November 7, 2019 — Real-world, four-year study results of the In.Pact Admiral drug-coated balloon (DCB) demonstrate long ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
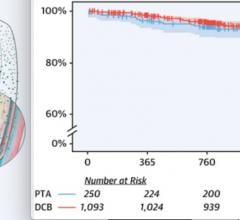
November 7, 2019 — There was no difference between drug-coated balloons (DCB) vs. plain old balloon angioplasty (POBA) ...
November 6, 2019 – Positive, two-year data from the first-in-human study of Selution SLR, MedAlliance’s novel sirolimus ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 6, 2019 – Boston Scientific announced positive data for two of its devices within the peripheral drug-eluting ...
October 15, 2019 — Royal Philips introduced two new balloons to its Stellarex 0.035-inch low-dose drug-coated balloon ...
October 10, 2019 — An independent analysis of Lutonix 035 drug-coated balloon (DCB) patient-level data showed no ...
Chandan Devireddy, M.D., offers insights about what he saw as the top take aways from the 2019 Transcatheter ...
September 20, 2019 — Orchestra BioMed Inc., in partnership with Terumo Corp. announced the company has secured ...
August 27, 2019 — Concept Medical was recently granted Breakthrough Therapy designation by the U.S. Food and Drug ...
August 14, 2019 — Concept Medical Inc. (CMI) has been granted "Breakthrough Device Designation" from the U.S. Food and ...
August 2, 2019 — B. Braun Interventional Systems Inc. (BIS) announced the U.S. Food and Drug Administration (FDA) has ...
July 15, 2019 — Edwards LifeSciences is recalling the IntraClude Intra-Aortic Occlusion Device due to a risk of balloon ...
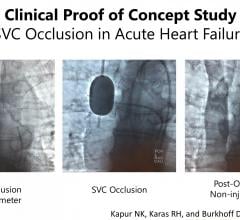
June 17, 2019 – Results from a first-in-man proof of concept study found occlusion of the superior vena cava (SVC) ...
June 13, 2019 — Orchestra BioMed Inc. announced it has formed a global strategic partnership with Terumo Corp. for ...
May 29, 2019 — Philips announced the three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European ...


 November 07, 2019
November 07, 2019