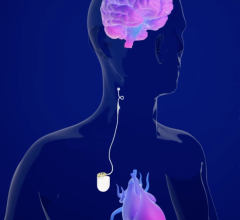
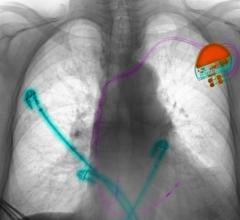
May 18, 2024 — Boston Scientific Corporation today announced positive six-month results from the ongoing pivotal MODULAR ATP clinical trial of the mCRM System,* the first modular cardiac rhythm ...
Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.
Oct. 10, 2025 — Berlin Heals Holding AG, a clinical-stage medical device company focused on revolutionizing the care of ...
June 13, 2024 — Xeltis, a leading developer of transformative implants that enable the natural creation of living and ...
June 12, 2024 — Royal Philips, a global leader in health technology, announced the first implant of the Duo Venous Stent ...
May 18, 2024 — Boston Scientific Corporation today announced positive six-month results from the ongoing pivotal MODULAR ...
February 12, 2023 — A team at Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital has successfully ...
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
July 3, 2023 — Nearly one-third of patients with an implanted device to prevent sudden death have anxiety in the first ...
February 24, 2023 — Biotronik announced that it has received CE approval for the Selectra 3D implant tools to include ...
January 3, 2023 — The market has been studied for the below mentioned-segmentation and regional analysis for North ...
November 22, 2022 — CroíValve has announced the successful First in Human implants of its DUO Tricuspid Coaptation Valve ...
November 2, 2022 — For decades, left ventricular-assist devices (LVADs) have extended the lives of people whose hearts ...
August 5, 2022 — Black people and women with severe heart failure who might be good candidates for surgery to implant a ...

The current standard of care for heart failure (HF) is guideline-directed medical therapy combined with device-based ...
June 16, 2020 - Biotronik has today announced its commitment to giving physicians additional tools to pace in the His ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...





 October 13, 2025
October 13, 2025