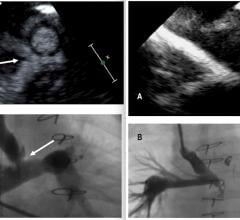
Nov. 17, 2025 — GE HealthCare has announced that Cardiovascular Associates of America (CVAUSA) plans to broaden its adoption of GE HealthCare’s FDA-approved cardiac positron emission tomography (PET) ...
Contrast Media
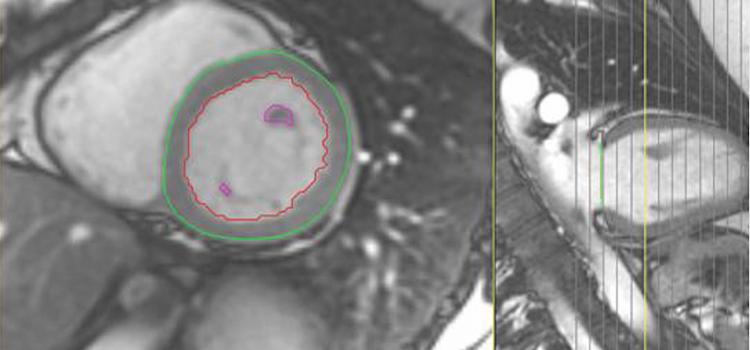
Contrast media, also called contrast agents, are used to enhance the blood and perfusion in tissues.This includes iodine based contrast for on computed tomography (CT), gadolinium based agents for MRI and lipid bubble contrast agents used in ultrasound.
Feb. 12, 2026 — ACIST Medical Systems, Inc., a Bracco Group company, has announced the launch in selected markets in ...
Nov. 17, 2025 — GE HealthCare has announced that Cardiovascular Associates of America (CVAUSA) plans to broaden its ...
March 27, 2025 — GE HealthCare is announcing the U.S. launch of Flyrcado (flurpiridaz F 18) injection at the 2025 ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Did you know that you have instant access to the latest webinars from Diagnostic and Interventional Cardiology?
DAIC ...
On Jan. 10, 2024, Diagnostic and Interventional Cardiology presented a webinar on "Contrast Management in Modern PCI."
...
On Jan. 10, 2024, Diagnostic and Interventional Cardiology presented a webinar on "Contrast Management in Modern PCI."
...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 15, 2023 — Contrast enhanced ultrasound (CEUS) is underutilized in the United States, and reduced access to ...
August 17, 2023 — University of Missouri School of Medicine neurologist Adnan Qureshi, MD recently led a study that ...
As part of the Consolidated Appropriations Act of 2018, pass-through payment status for LUMASON® (sulfur hexafluoride ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 29, 2021 – A new original science category being featured at the American Society of Echocardiography (ASE) 2021 ...
June 29, 2021 – Echocardiography with ultrasound enhancing agents (UEAs) has proven to be a valuable imaging procedure ...
May 12, 2021 — According to the British Heart Foundation, heart and circulatory diseases cause more than a quarter (27 ...

 February 12, 2026
February 12, 2026