October 4, 2019 – RenalGuard Therapy was found to be superior to the POSEIDON method in preventing contrast-induced acute kidney injury (CI-AKI) for patients with kidney disease who undergo interventional cath lab procedures. Results of the 700 patient, randomized, investigator-driven REMEDIAL III Trial presented at the 2019 Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
The contrast used in interventional procedures can injure the kidney in a number of ways, including direct toxicity, blocking oxygen delivery to the kidney and increasing fluid loss. RenalGuard Therapy is designed to protect patients by inducing and maintaining high urine output. A number of clinical trials found that the high urine output induced by RenalGuard Therapy reduces the incidence of CI-AKI.
“Acute kidney injury can be a serious complication due to the contrast received during invasive diagnostic or interventional procedures,” said Carlo Briguori, M.D., PhD, Chief of the laboratory of interventional cardiology at the Mediterranea Cardiocentro in Naples, Italy. “Hydration is the cornerstone to prevent this complication. However, uncontrolled hydration may lead to acute pulmonary edema. The REMEDIAL III trial compared two tailored hydration regimens to reduce these risks as a whole in high risk patients. The study found that UFR-guided hydration is superior to LVEDPguided hydration to prevent acute kidney injury and acute pulmonary edema. The number needed to treat to prevent one event with the RenalGuard system was 22.”
Contrast-induced acute kidney injury is a powerful predictor of unfavorable early and late outcomes, according to the authors of the REMEDIAL II trial. CI-AKI also accounts for approximately 10 percent of all causes of hospital-acquired renal failure and increases length of stay.
The REMEDIAL III trial was designed to evaluate the ability of the two most successful patient “tailored hydration regimens” to protect at-risk patients from CI-AKI by comparing RenalGuard Therapy with left ventricular end-diastolic pressure (LVDEP)-guided hydration (the “POSEIDON” method).
The data showed RenalGuard Therapy to be superior to LVEDP-guided hydration for the prevention of CI-AKI, as demonstrated by a significantly lower incidence of CI-AKI and/or pulmonary edema, and a lower incidence of major adverse events one month post-treatment. Overall 10.3 percent of patients (36/351) treated with LVDEP-guided hydration developed CI-AKI and/or pulmonary edema, compared with 5.7 percent (20/351) of patients treated with RenalGuard Therapy. One month post-treatment, 12 percent of patients (44/351) treated with LVDEP suffered major adverse events including dialysis, sustained kidney damage, acute pulmonary edema, and death vs. 7.1 percent (21/351) treated with RenalGuard Therapy.
The investigators concluded that RenalGuard Therapy is superior to the LVEDP-guided hydration regimen to prevent the composite of CI-AKI and/or acute pulmonary edema in high-risk patients.
The vendor said RenalGuard Therapy technology has now been used with more than 23,000 patients.
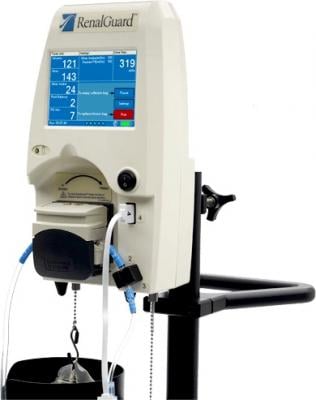
RenalGuard Therapy measures a patient’s urine output and automatically adjusts hydration on an individual basis to induce high urine rates, which have been shown to protect the kidney from a range of insults. The system has been used with more than 23,000 patients. A growing body of clinical evidence demonstrates RenalGuard’s ability to protect patients from AKI and CIN following catheterization procedures when compared to the standard of care. RenalGuard is CE-marked and is sold in Europe and other countries via a network of distributors.
Find information on other late-breaking TCT trials
Related CIN Content:
Sliding Scale Hydration Washes Out Contrast-Induced Kidney Complications
Contrast-Induced Kidney Injury Prevented With RenalGuard in REMEDIAL III Trial
VIDEO: How to Avoid Acute Kidney Injury in the Cath Lab — Interview with Hitinder Gurm, M.D.
VIDEO: Strategies to Avoid Acute Kidney Injury Caused by Cath Lab Contrast — Interview with Roxana Mehran, M.D.
VIDEO: Combating Contrast Induced Nephropathy in the POSEIDON Trial — Presntation by Sonjot Brar, M.D.


 October 24, 2025
October 24, 2025 









