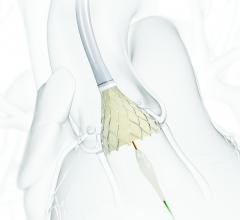
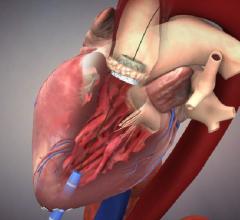
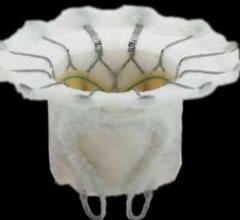
August 5, 2015 — Abbott announced Monday that it has entered into an agreement to purchase Tendyne Holdings Inc., a ...
Hybrid OR
This channel includes news and new technology innovations connected with hybrid operating rooms, also referred to as hybrid ORs, hybrid cath labs or hybid interventional suites. These rooms combine catheterization lab and OR technologies and requirements fro both open surgical and transcatherer procedures.
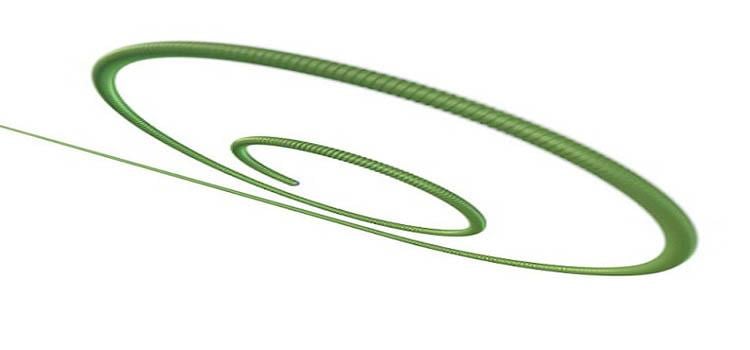
July 28, 2015 — Boston Scientific announced CE Mark and U.S. Food and Drug Administration (FDA) clearance for the Safari ...
Interventional cath labs use X-ray angiography imaging systems to guide catheters through blood vessels to perform ...
July 21, 2015 — The recent U.S. Food and Drug Administration (FDA) approvals of two next-generation transcatheter aortic ...

July 17, 2015 - Building teams that include advanced practice providers can help cardiovascular practices meet the ...
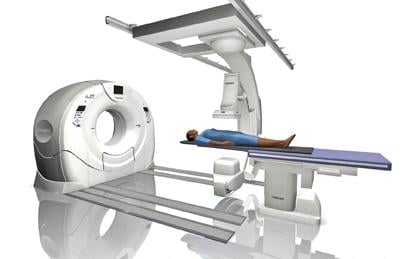
Angiographic imaging system vendors have developed several new technologies to address emerging cath lab trends ...

July 1, 2015 - CardiAQ Valve Technologies (CardiAQ) announced that its second-generation transcatheter bioprosthetic ...
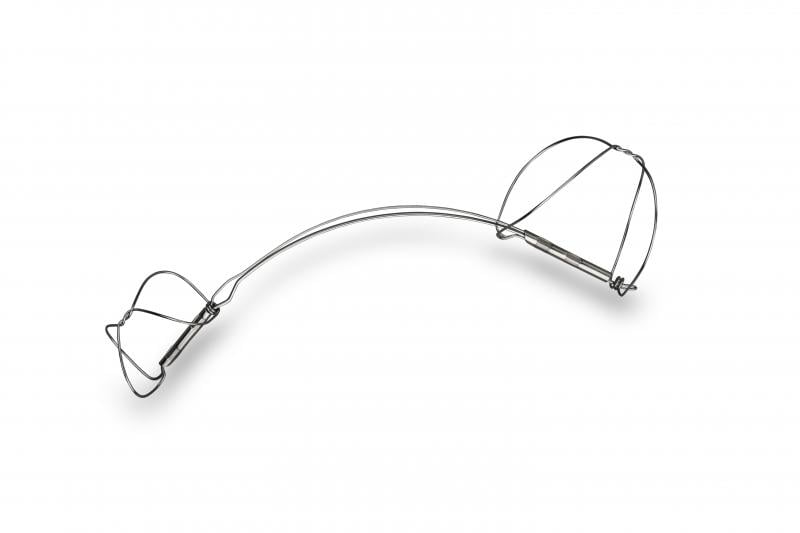
June 30, 2015 - Cardiac Dimensions announced the first patients have been enrolled in the REDUCE FMR clinical trial ...
June 23, 2015 - Medtronic plc announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the ...
June 19, 2015 - Edwards Lifesciences Corp. announced U.S. Food and Drug Administration (FDA) approval of the Edwards ...
June 18, 2015 - IMRIS Inc. received a letter May 26 from the NASDAQ Stock Market stating that it would be delisted and ...
Interview with Juan Granada, M.D., executive director and chief scientific officer of the Cardiovascular Research ...
April 7, 2015 — IMRIS Inc. announced that Visius iCT, its ceiling-mounted intraoperative computed tomography scanner ...
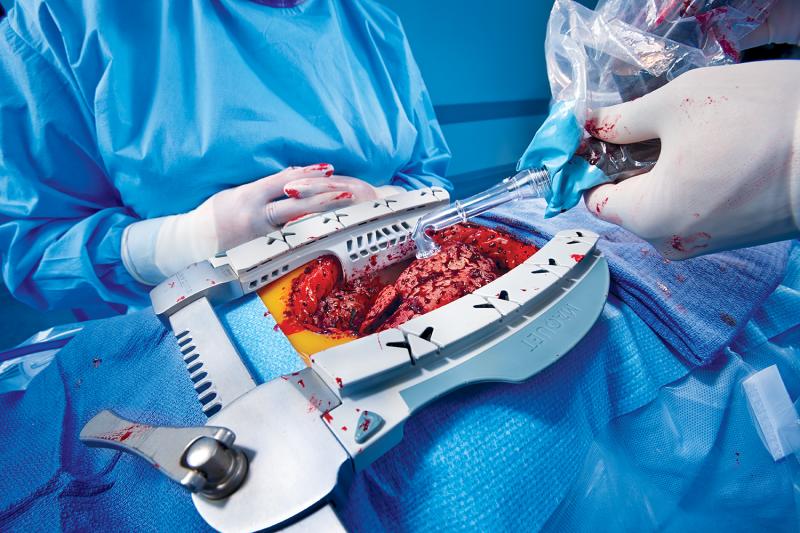


 August 05, 2015
August 05, 2015