Hybrid OR
This channel includes news and new technology innovations connected with hybrid operating rooms, also referred to as hybrid ORs, hybrid cath labs or hybid interventional suites. These rooms combine catheterization lab and OR technologies and requirements fro both open surgical and transcatherer procedures.

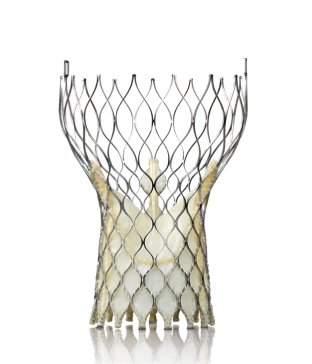
October 30, 2013 — The first results from the CoreValve U.S. pivotal trial, the first U.S. data presented on the ...
October 10, 2013 — IMRIS Inc. and Siemens Healthcare announced an agreement that positions one of Siemens’s computed ...
October 4, 2013 — Eizo Corp. and Stryker Corp. announced their partnership to offer customers integrated large monitor m ...

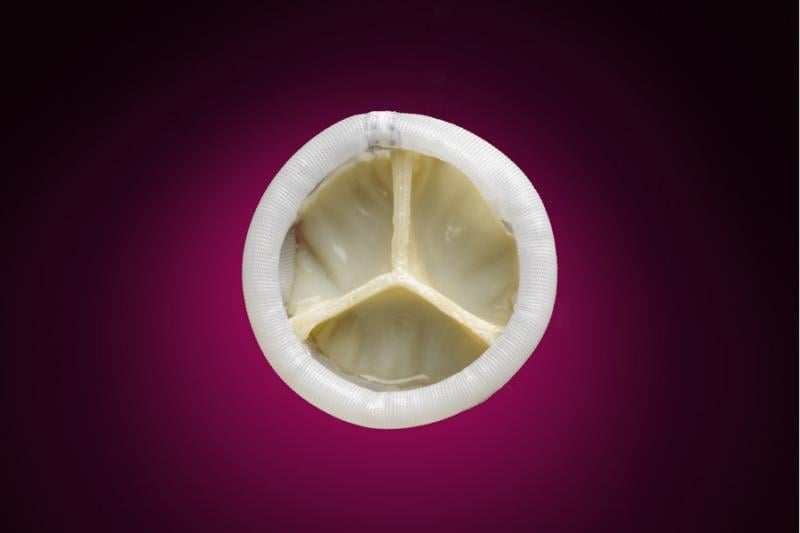
September 11, 2013 — Direct Flow Medical Inc. announced the first patient enrollment in the U.S. SALUS clinical trial ...
August 13, 2013 — IMRIS Inc. has obtained regulatory CE mark for Visius iCT, the first and only ceiling-mounted ...
July 23, 2013 — IMRIS has announced U.S. Food and Drug Administration (FDA) 510(k) clearance to market VISIUS iCT, the ...

July 12, 2013 — Virginia Anderer, a Chicago native living in southern California, was the first patient in San Diego and ...
July 9, 2013 — Researchers have announced the results of a clinical study that shows a key difference in the patient’s ...
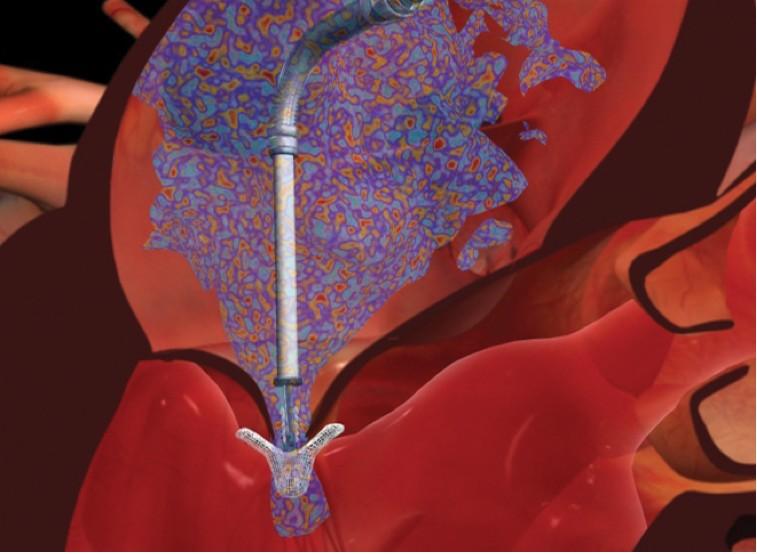
June 13, 2013 — Abbott announced publication of positive outcomes from two European post-approval studies of the ...

June 10, 2013 — Direct Flow Medical Inc. announced that it has received approval from the U.S. Food and Drug ...

June 3, 2013 — Medtronic Inc. announced it has received CE mark for valve-in-valve (VIV) procedures using the CoreValve ...

 October 31, 2013
October 31, 2013