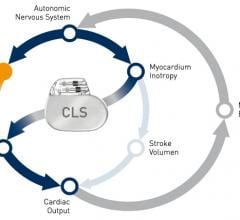
May 9, 2016 — Medtronic announced the results of several studies evaluating a novel approach to implantable cardioverter ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
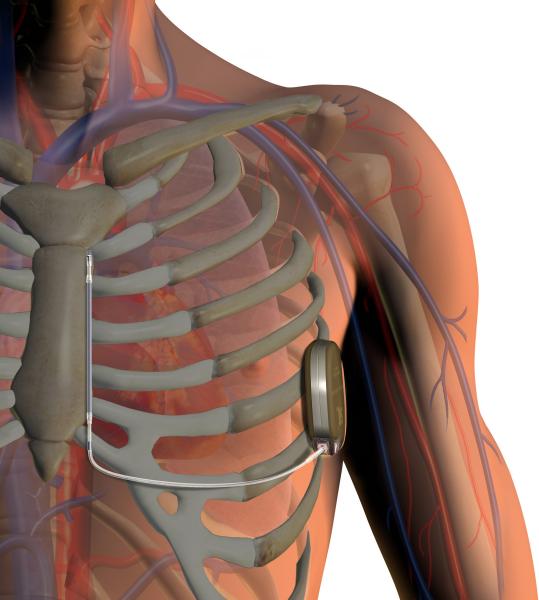
May 9, 2016 — The mid to long-term results from the EFFORTLESS study, the largest subcutaneous implantable cardioverter ...
April 3, 2016 — Many patients who have an implantable cardioverter defibrillator (ICD) are unaware the device can be ...
March 17, 2016 — Medtronic plc announced that it received CE (Conformité Européenne) Mark for the first and only cardiac ...
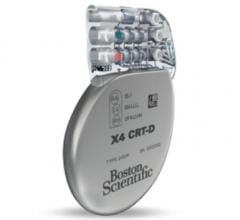
March 4, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for the Acuity X4 ...
February 25, 2016 — Biotronik announced that its Itrevia 7 HF-T cardiac resynchronization therapy defibrillator (CRT-D) ...
February 3, 2016 — Biotronik announced CE approval for its new Ilivia implantable cardioverter defibrillators (ICDs) and ...
January 26, 2016 — St. Jude Medical Inc. is recalling the Optisure implantable cardioverter defibrillator (ICD) leads ...
December 22, 2015 - The U.S. Food and Drug Administration has cleared the Zoll LifeVest Wearable Cardioverter ...
December 22, 2015 — Biotronik received U.S. Food and Drug Administration (FDA) approval for use of its latest family of ...
December 14, 2015 — St. Jude Medical Inc. announced CE Mark approval for magnetic resonance (MR) conditional labeling ...
December 8, 2015 — At Ahuja Medical Center, University Hospitals (UH) cardiologists recently implanted the Boston ...
December 8, 2015 — Biotronik announced the first patient enrollments to the B3 clinical trial. The study will evaluate ...
November 4, 2015 — LivaNova PLC, formerly Sorin Group, launched Platinum, a new range of implantable cardiac ...
October 30, 2015 — Boston Scientific Corp. has received CE Mark on magnetic resonance imaging (MRI) conditional labeling ...

 May 09, 2016
May 09, 2016