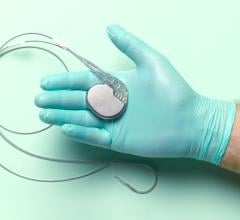
March 26, 2024 — UC San Diego Health is the first health system in San Diego to successfully implant the world’s first dual chamber and leadless pacemaker system to help treat people with irregular ...
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.
March 26, 2024 — UC San Diego Health is the first health system in San Diego to successfully implant the world’s first ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, BIOTRONIK proudly announces the world ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, Biotronik announces the world’s first ...
February 14, 2024 — The American College of Cardiology’s newest registry offers data-driven insights on cardiac ...
January 19, 2024 — Orchestra BioMed, a biomedical company accelerating high-impact technologies to patients through risk ...
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
January 5, 2024 — Medtronic, a global leader in healthcare technology, today announced it has received CE (Conformité ...
December 22, 2023 — TRiCares SAS (“TRiCares”), a privately held pioneer in the field of minimally invasive treatment of ...
September 20, 2023 — Orchestra BioMed Holdings, Inc., a biomedical company accelerating high-impact technologies to ...
August 21, 2023 — The first implant in Europe of Biotronik’s latest pacemaker and CRT-P generation was conducted in ...
July 5, 2023 — Research presented during the American Society of Echocardiography’s 34th Annual Scientific Sessions, ASE ...
June 1, 2023 — Every year more than one million people receive a pacemaker. Until now, leadless versions were only ...
May 25, 2023 — Abbott announced late-breaking results from the AVEIR dual-chamber (DR) i2i Investigational Device ...



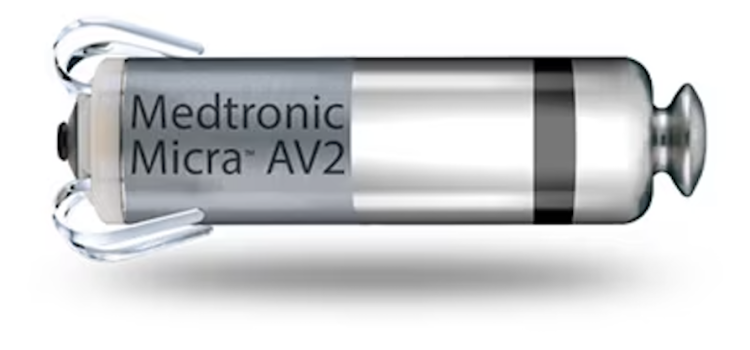


 March 26, 2024
March 26, 2024