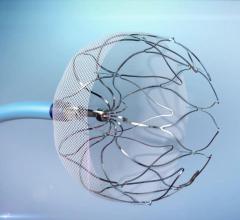
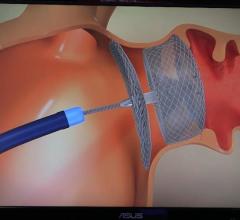
February 3, 2020 — The U.S. Food and Drug Administration (FDA) has approved a the CATALYST trial designed to assess ...
Structural Heart Occluders
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex
October 28, 2019 — The Gore Cardioform ASD Occluder received European CE mark clearance in early October. The device ...
 I recently had the opportunity to conduct an onsite visit to the University of Colorado Hospital Heart and Vascular ...
I recently had the opportunity to conduct an onsite visit to the University of Colorado Hospital Heart and Vascular ...
September 20, 2019 – Abbott announced approvals in Europe for two of its pediatric devices — the Masters HP 15mm ...
June 10, 2019 – W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket ...
This video offers an overview of the Watchman left atrial appendage (LAA) occluder system, including information of its ...
March 27. 2019 — A newly released expert consensus statement provides recommendations for the safe and effective ...
February 13, 2019 — Le Bonheur Children's Hospital cardiologists in Memphis, Tenn., implanted the Amplatzer Piccolo ...
January 16, 2019 — Abbott announced the U.S. Food and Drug Administration (FDA) approved the Amplatzer Piccolo Occluder ...
Robert Quaife, M.D., director of advanced cardiac imaging, University of Colorado Hospital, explains why advanced ...
Karen Orjuela, M.D., assistant professor of neurology at the University of Colorado Stroke and Brain Aneurysm Center, ex ...
Ashish Pershad, M.D., medical director, structural heart program, Banner University Medical Heart Institute, Phoenix ...
This is a demonstration of the the Philips TrueVue photo-realistic rendering and lighting source technology. This ...
April 3, 2018 — Following the unprecedented Gore REDUCE Clinical Study conclusion that closure of patent foramen ovale ...

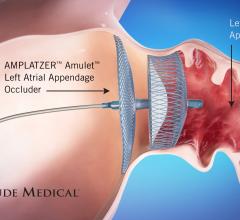
 February 03, 2020
February 03, 2020