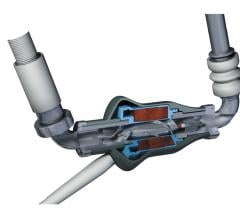
August 11, 2015 — HeartWare International Inc. announced that enrollment in the ENDURANCE2 destination therapy clinical ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
August 7, 2015 — Mayo Clinic is announcing results of a study on the effectiveness of left ventricular assist devices ...
August 6, 2015 — The U.S. Food and Drug Administration (FDA) is alerting healthcare providers, patients and caregivers ...
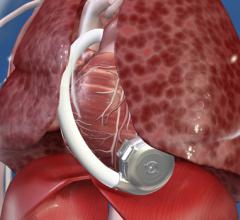
August 5, 2015 — HeartWare expanded the Class I recall of its ventricular assist device in mid-June due to concerns over ...
August 4, 2015 — Over the past several years, advanced heart failure patients who have not responded to medical therapy ...

July 22, 2015 — St. Jude Medical and Thoratec announced that the boards of directors of both companies have unanimously ...
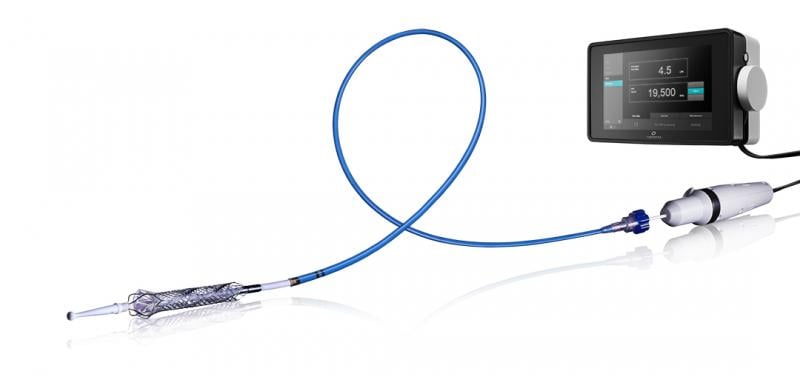
July 15, 2015 - Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval ...
July 9, 2015 - During an event with Massachusetts Gov. Charlie Baker in June, Abiomed announced a major expansion of its ...
June 11, 2015 - ECRI Institute recently reviewed published data on Abiomed's Impella RP (Right Percutaneous) ...
June 9, 2015 - University Hospitals Case Medical Center physicians in the Harrington Heart & Vascular Institute were the ...
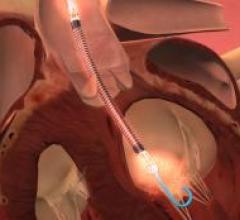
June 4, 2015 - Beaumont Hospital, Royal Oak is one of the first centers in the United States to use a new minimally ...
May 4, 2015 — Thoratec Corp. announced results from the ROADMAP Study (Risk Assessment and Comparative Effectiveness of ...
April 27, 2015 — CardiacAssist Inc. announced that it received a Class 3 CE-mark for its Protek Duo veno-venous cannula ...
April 9, 2015 — Maquet Cardiovascular USA announced publication of a manuscript comparing the clinical and economic ...

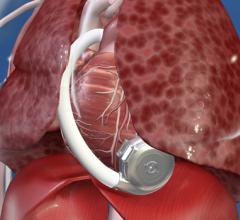
 August 11, 2015
August 11, 2015