December 21, 2007 - Edwards Lifesciences Corp. (Edwards) has completed its acquisition of certain assets of the ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
December 19, 2007 - Abiomed Inc. today announced that it has received approval from the FDA for U.S. commercial ...
December 13, 2007 - World Heart Corp., a developer of implantable mechanical circulatory support systems for ...
December 13, 2007 - The American Red Cross and the American Heart Association join in thanking Congress today for ...
December 4, 2007- Berlin Heart announced the first patient enrollment in the prospective IDE study for its EXCOR ...
November 21, 2007 - Ventracor announced 10 U.S. hospitals have implanted at least one VentrAssist left ventricular ...
CircuLite Inc. reported the launch of the clinical development program for its Synergy Pocket Circulatory Assist ...
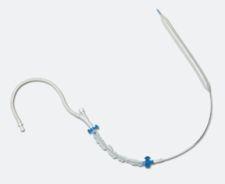
A study published in 2002, which assigned a 0-5 score to various clinical parameters, is still considered a major ...
November 4, 2007 - A small, implantable device that helps the heart pump blood works equally well for men and women but ...
October 25, 2007 - Abiomed Inc. announced for the first time, at TCT 2007, that the Impella 2.5 PROTECT I safety trial ...
October 16, 2007 - Abiomed Inc. will be presenting clinical data on Impella 2.5 percutaneous Ventricular Assist Device ...
September 18, 2007 - The DuraHeart Left Ventricular Assist System (LVAS) reported a survival rate of 77 percent for all ...
August 30, 2007 - Abiomed Inc. announced it has received conditional approval from the FDA to begin its pivotal clinical ...
August 30, 2007 - Concentric Medical announces that it has obtained FDA 510(k) clearance for its Merci L6 Retriever, a ...
August 15, 2007 - Thoratec Corp., a world leader in products to treat cardiovascular disease, said today that its ...

 December 26, 2007
December 26, 2007
