The U.S. Food and Drug Administration (FDA) sent a letter to cardiologists this week to explain its evaluation of high ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
Mark Anderson, M.D., FACS, vice chair of cardiac surgery services and cardiothoracic surgeon at Hackensack University ...
Michael Amponsah, M.D., FACC, an interventional cardiologist at Mohawk Valley Health System, shares a case of Impella CP ...
December 11, 2018 — The scientific journal Nature recently published an article from Munich University Hospital which ...
Navin Kapur, M.D., discusses the results of the FDA STEMI Door-to-Unloading (DTU) safety and feasibility randomized ...
Michael Flaherty, M.D., discusses a study published in Circulation Research which finds that use of hemodynamic support ...

October 19, 2018 — Abbott announced today that the HeartMate 3 Left Ventricular Assist Device (LVAD) has received U.S ...
Nevin Kapur, M.D., FAHA, FACC, FSCAI, executive director, Cardiovascular Center for Research and Innovation, Tufts ...
September 19, 2018 — Abiomed Inc. announces its initiatives at the 30th Transcatheter Cardiovascular Therapeutics (TCT) ...
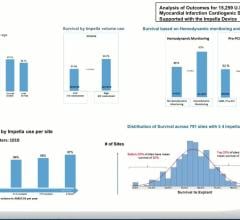
William O’Neill, M.D., outlines his recent clinical publication of AMICS patients from the Impella Quality (IQ) database ...
Behnam Tehrani, M.D., FSCAI, director of the cardiac cath lab, INOVA Heart and Vascular Institute, Fairfax, Va ...
July 17, 2018 — Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for a less-invasive ...
July 11, 2018 — French-based CorWave announced that its CALYPSO program has received 14 million euros to develop CorWave ...

About 50 percent of patients in cardiogenic shock do not survive, and account for about 90,000 heart attack patients a ...

 February 05, 2019
February 05, 2019