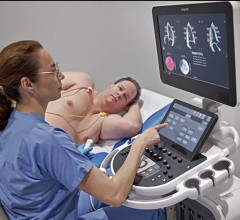
January 13, 2023 — A change to Medicare policy surrounding heart transplant may lead to increased inequities in access to transplant for patients with heart failure, a Michigan Medicine study finds ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
June 19, 2024 — When electrophysiologist Eugenio Cingolani, MD, isn’t seeing patients, he can usually be found in his ...
February 21, 2024 — Ochsner Children's Hospital, ranked among the top hospitals in the nation for pediatric cardiology a ...
October 31, 2023 — Tenaya Therapeutics, Inc., a clinical-stage biotechnology company with a mission to discover, develop ...
October 5, 2023 — A newly-issued "Scientific Statement on Durable Mechanical Circulatory Support" in the October issue ...
September 15, 2023 — A team of interventional cardiologists from Henry Ford Health’s Center for Structural Heart Disease ...
July 14, 2023 — In recent years, transcatheter intervention techniques have emerged as a promising alternative for the ...
June 7, 2023 — Magenta Medical Ltd. has announced the initiation of its FDA-approved Early Feasibility Study with the ...
March 1, 2023 — Mesoblast Limited, global leader in allogeneic cellular medicines for inflammatory diseases, today ...
January 13, 2023 — A change to Medicare policy surrounding heart transplant may lead to increased inequities in access ...
November 2, 2022 — For decades, left ventricular-assist devices (LVADs) have extended the lives of people whose hearts ...
November 1, 2022 — Abiomed announced that Impella RP Flex with SmartAssist has received U.S. Food and Drug ...
October 12, 2022 — Following the recent European Society of Cardiology (ESC) Congress in Barcelona, Spain last month, ...
September 17, 2022 — Results of a new per-protocol analysis of the ST-segment Elevation Myocardial Infarction Door-To ...
August 25, 2022 — The U.S. Food and Drug Administration (FDA) issued a statement that Medtronic, Inc. is recalling Heart ...

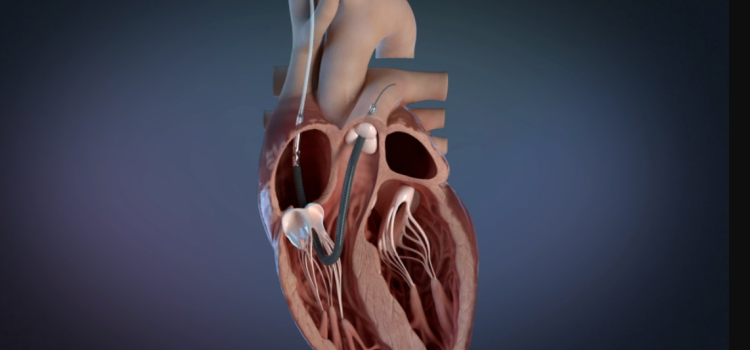

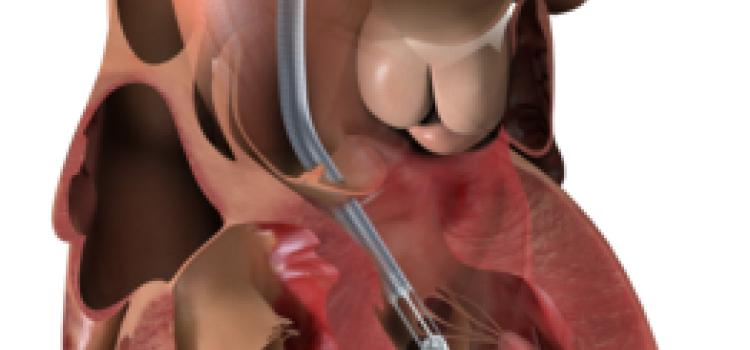

 June 19, 2024
June 19, 2024