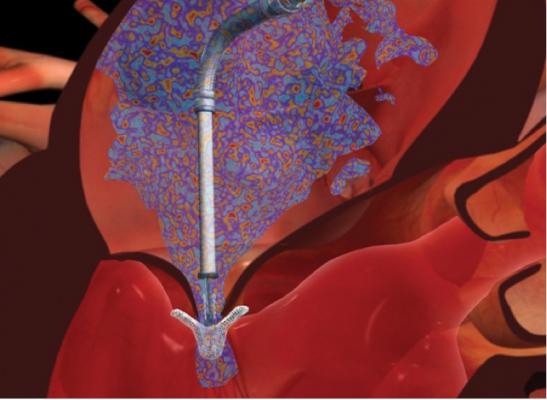
February 29, 2016 — Abbott has initiated a voluntary safety notice regarding the MitraClip Delivery System to reinforce the proper procedures used to operate and deploy the device. The company received a small number of reports involving MitraClip Delivery Systems where the user was unable to separate the implantable clip from the delivery system. Presently, there are 3,534 devices on the market (1,288 in the United States and 2,246 outside the United States). Abbott has received nine Medical Device Reports of malfunction.
Abbott's investigation determined that the delivery system's "arm positioner" was not returned to the required neutral position by the operator during the deployment sequence, subsequently preventing the clip from detaching. All of these cases (0.17 percent incidence) resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem. There was one patient death in these cases as a result of severe comorbidities following surgery.
The U.S. Food and Drug Administration (FDA) has classified this safety notice as a Class I Recall. Abbott is not removing product from commercial distribution. Abbott is requiring training of all MitraClip-implanting physicians on the safety notice to ensure continued safe use of the device, and is incorporating the safety notice's deployment sequence in its Instructions for Use.
Commercial clip delivery systems with lot numbers 50714U1 and greater are within the scope of this action. Individuals who have already had the device implanted are not affected by this action.
The MitraClip Delivery System is a minimally invasive device used to treat people with degenerative mitral regurgitation (DMR), a condition involving a dysfunction of the heart's mitral valve. Approved by the FDA in 2013 for patients at high risk for surgery, the device demonstrated favorable outcomes in a study presented at the American College of Cardiology (ACC) 2015 scientific session, the first outcomes study outside of a clinical trial. Five hundred sixty-four patients were treated with the MitraClip device, with 93 percent of patients achieving a mitral regurgitation grade of less than or equal to 2, with 63.6 percent at a grade of less than or equal to 1, demonstrating a significant decrease in leakage. Adverse events and procedural complications were low.
For more information: www.fda.gov/medwatch


 November 14, 2025
November 14, 2025 









