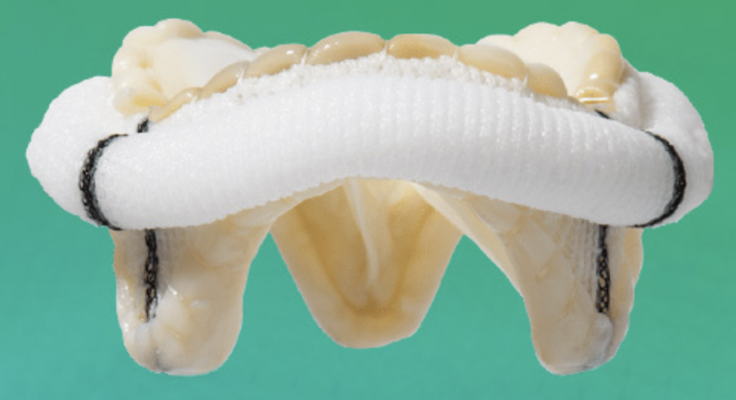
March 31, 2023 — Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the company's Epic Max stented tissue valve to treat people with aortic regurgitation or stenosis. This device is the latest addition to Abbott's Epic surgical valve platform which has a decades-long history of safety and strong clinical outcomes, with an optimized design to further improve valve blood flow.
When the aortic valve doesn't close properly (aortic regurgitation) or fails to open fully (aortic stenosis), the heart doesn’t pump blood efficiently and blood flow to the body is reduced. If left untreated, aortic valve disease can lead to heart failure, stroke, blood clots or death. Diseased or damaged heart valves that can't be repaired may be surgically replaced with either mechanical or bioprosthetic (tissue) valves in an open-heart surgical procedure. Bioprosthetic valves like Epic Max are recommended for patients requiring valve replacement who aren't suitable for taking blood-thinning medication.
Epic Max is designed to achieve excellent hemodynamics, or blood flow, and its low-profile frame facilitates potential future transcatheter interventions for patients. This new valve is built on the Epic surgical valve platform, leveraging its long-term performance and durability.
"The aortic valve is one of the heart valves most commonly impacted by cardiovascular disease, frequently requiring replacement," said Joseph E. Bavaria, M.D., cardiovascular surgery, University of Pennsylvania, "Abbott's Epic Max design optimizes blood flow for patients and has a low profile that makes future cardiac interventions, if necessary, easier."
"With Epic Max, we're accomplishing two important things: First and foremost, we're improving heart valve hemodynamics, which is the purpose of the procedure. Secondly, we're preserving options and ability for patient lifetime disease management, an ever more critical point of consideration in device therapy selection," said Michael Dale, senior vice president of Abbott's structural heart business.
For more information: https://abbo.tt/EpicMaxISI


 July 08, 2024
July 08, 2024 









