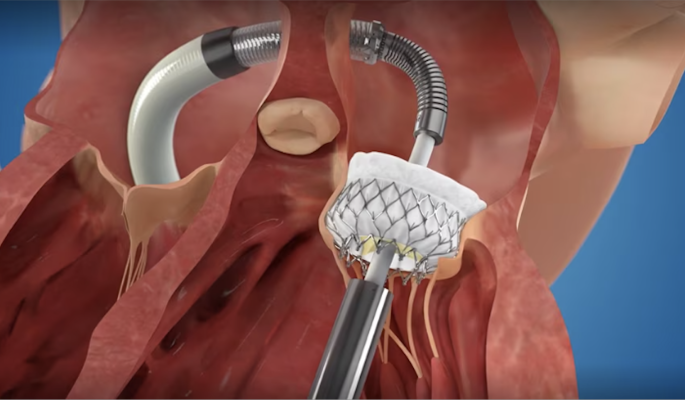
Pictured is the TMVR procedure
February 20, 2024 — As New Jersey’s leading provider of high-quality cardiac procedures and diagnostic testing and an academic institution, Hackensack Meridian Jersey Shore University Medical Center provides patients with state-of-the-art clinical trials in the management of heart valve disease and congestive heart failure. The academic medical center recently announced its participation in the Medtronic APOLLO Clinical Trial, which is evaluating the safety and efficacy of an investigative device called the Intrepid Transcatheter Mitral Valve Replacement System (TMVR) for moderate-to-severe or severe symptomatic mitral regurgitation (MR) in patients who may be unsuitable for approved transcatheter repair or surgery. It is one of only two New Jersey hospitals offering the trial to patients.
“Many patients with symptoms of mitral regurgitation are not candidates for open heart surgery due to their advanced age or competing comorbidities, nor are they candidates for the only alternative, the MitraClip device. They are left with no options other than medicines. The Intrepid TMVR is a heart valve that is designed to help alleviate mitral regurgitation so that blood can flow through the heart in the intended direction,” said Matthew Saybolt, M.D., FACC, principal site investigator and medical director, Structural Heart Disease Program, Jersey Shore University Medical Center. “It is a complete mitral valve replacement that can be implanted minimally invasively through a patient's femoral vein, through a catheter or tube.”
Your heart has four valves that move blood in and out of its four chambers. One of the valves is called the mitral valve. When a mitral valve is functioning normally, it regulates blood flow from the upper chamber (left atrium) of your heart to the lower chamber of your heart (left ventricle). However, when the mitral valve is diseased, it may not close tightly and blood can leak back into the left atrium, where it came from. This is known as a "leaky valve" or MR.
Mitral valve disease has many causes, such as heart attack and other types of heart disease, a heart condition present at birth, certain infections, or simply older age. “People may not have symptoms for many years, if at all, but they can include; fatigue, irregular heart sound or murmur, irregular heartbeat and shortness of breath,” said Dr. Saybolt.
Individuals or physicians interested in more information, or for a referral to the program, should contact Dr. Saybolt and the cardiac team at 732-776-4973 or email [email protected].
To view information about the multi-center, global, prospective, non-randomized, interventional, pre-market trial on the U.S. National Library of Medicine website, visit https://clinicaltrials.gov/ct2/show/NCT03242642. For more information about the Intrepid Transcatheter Mitral Valve Replacement System (TMVR), visit https://www.medtronic.com/us-en/patients/clinical-trials/apollo.html.
“The expertise of our clinicians, like Dr. Saybolt, enables our academic medical center to provide these leading-edge clinical trials. I’m glad we are able to offer this expert care to the community we serve,” said Vito Buccellato, MPA, LNHA, president and chief hospital executive, Jersey Shore University Medical Center.
Jersey Shore University Medical Center continues to receive high-quality accolades recognizing its cardiovascular care. The American College of Cardiology (ACC) has recognized the medical center with the HeartCARE Center National Distinction of Excellence, Cardiac Cath Lab Accreditation with Percutaneous coronary intervention (PCI), Chest Pain Center with Primary PCI and Resuscitation Accreditation, Heart Failure Accreditation with Outpatient Services and the NCDR Chest Pain ̶ MI Registry Platinum Performance Achievement Award.
“Monmouth and Ocean county residents are becoming increasingly aware of the world-class cardiovascular care provided at Jersey Shore. Our program continues to grow as more of our neighbors select the academic medical center to receive exemplary health care,” said Kenneth N. Sable, M.D., MBA, FACEP, regional president, Southern Market, Hackensack Meridian Health.
For more information: www.hmhn.org


 February 03, 2026
February 03, 2026 









