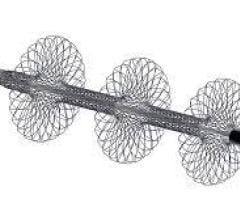
October 14, 2022 — Medtronic, a global leader in medical technology, announced the 36-month final results from the ABRE clinical study. The purpose of the ABRE clinical study was to evaluate the safety and effectiveness of the Abre venous self-expanding stent system, intended for the treatment of symptomatic iliofemoral venous outflow obstruction.
The study results were presented in a late-breaking clinical trial session at the American Vein and Lymphatic Society (AVLS) 2022 annual meeting. Professor Stephen Black, M.D., consultant vascular surgeon, Guy's and St. Thomas' Hospital, London and co-principal investigator for the ABRE Study, presented the data.
“The 36-month ABRE data has continued to demonstrate the long-term durability of interventions in patients suffering from deep venous disease,” Professor Black said. “The results show a sustained result in both technical aspects, but more importantly, in patient outcomes.”
The ABRE Study included a complex set of patients. Within this patient group:
· 47.5% of subjects were categorized as having post-thrombotic syndrome (PTS),
· 35.8% of PTS subjects presented with a complete venous occlusion confirmed by the core lab
· Mean lesion length of subjects was 112.4 mm
· 44.0% of subjects had stents that extended below the inguinal ligament.
Of note, the study results showed:
· Overall, effectiveness following treatment with the Abre venous stent was sustained through 36 months as evidenced by a Kaplan-Meier estimated primary patency rate of 81.6% and a Kaplan-Meier estimated freedom from clinically driven target lesion revascularization (CD-TLR) rate of 89.3%.
· No stent fractures or delayed stent migrations were reported through 36 months.
· Sustained and clinically meaningful improvements were observed through 36 months compared to baseline as measured by EQ-5D and VEINES-QoL quality of life.
· Sustained and clinically meaningful improvements through 36 months as measured by Villalta and VCSS venous functional assessments indicates less severity of PTS disease and venous disease overall.
“Medtronic is strongly committed to the deep venous market. As the 36-month results show, the Abre venous stent system safely and effectively treats venous disease,” said Dave Moeller, president of the Peripheral Vascular Health Operating Unit at Medtronic. “This study included many patients with highly challenging cases, showing that Abre is meaningfully able to improve patients’ quality of life — including those with severe disease — for a long period.”
For more information: www.medtronic.com
Related Content:
Medtronic Launches IDE Study of Abre Venous Self-Expanding Stent
Medtronic Venous Stent Receives U.S. FDA Approval to Treat Venous Outflow Obstruction

 October 27, 2025
October 27, 2025