October 22, 2013 — AngioScore Inc., a developer of
angioplasty catheters for use in the treatment of cardiovascular disease, announced that preliminary data from the
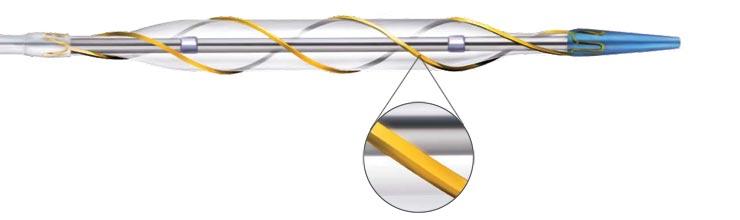
first-in-human (FIH) study (“Patent-C”) of the drug-coated AngioSculpt Scoring Balloon
Catheter will be presented at the Transcatheter Cardiovascular Therapeutics (TCT) Conference in San Francisco the week of Oct. 28.
This FIH trial enrolled 61 patients with coronary in-stent restenosis (ISR) at five international sites: four in Germany and one in Brazil. Bruno Scheller, M.D. and professor of interventional cardiology at Saarland University Hospital, Homburg, Germany, led the study, and along with Ulrich Speck of the Charité Hospital in Berlin, is one of the pioneers in the rapidly developing field of drug-coated balloons for the treatment of coronary and peripheral artery disease.
The drug-coated AngioSculpt FIH study was designed as a randomized controlled trial comparing the recently developed drug-coated AngioSculpt with the commercially available uncoated version of the AngioSculpt in patients presenting with significant restenosis in a previously implanted coronary bare metal stent. Patients underwent follow-up coronary angiography at six months to compare the rate of recurrent restenosis and late lumen loss (LLL) in both treatment arms. Additional study endpoints include the rate of major adverse cardiovascular events (MACE), clinically driven target lesion revascularization (TLR) and stent thrombosis for up to two years following the index procedure. All angiograms were analyzed by an independent core laboratory at the Cardiovascular Research Foundation in New York City.
The results of the FIM Study will be presented on the following date and times:
- Oct. 28, 7:45 p.m. — Drug-coated AngioSculpt FIM Clinical Trial Results, “Featured Clinical Research Session,” Bruno Scheller — Moscone Convention Center West room 2002
- Oct. 30, 7:00 a.m. — AngioScore Breakfast Symposium, Moscone Convention Center North room 121
- Oct. 31, 9:52 a.m. — Drug-coated AngioSculpt FIM Clinical Trial Results - “Spotlight Session – Drug Coated Balloons,” Alexandre Abizaid, Moscone Convention Center North room 135
The AngioSculpt Scoring Balloon Catheter is an investigational device limited by applicable law to investigational use and not available for sale.
For more information: www.angioscore.com


 June 13, 2024
June 13, 2024 









