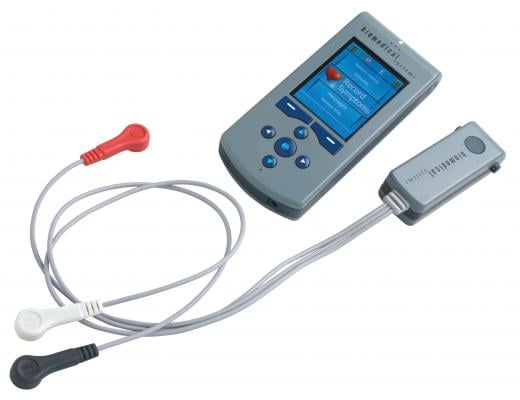
November 15, 2012 — The U.S. Patent and Trademark Office has awarded Biomedical Systems U.S. Patent Number 8301236 for its TruVue Wireless Ambulatory ECG Monitoring System. Biomedical Systems developed TruVue for the diagnosis and management of atrial fibrillation, considered the leading preventable cause of stroke affecting nearly three million Americans at a cost of $26 billion each year. The Centers for Disease Control and Prevention estimates the number of people impacted by atrial fibrillation to grow five times the current rate by 2050.
The patented TruVue technology records and wirelessly transmits every heartbeat for up to 30 days. Biomedical Systems houses the ECG information in its secure data center where certified cardiac technicians monitor and analyze abnormalities, post real-time reports and alert physicians to cardiac events. At any time, physicians can directly access information online — without working through support staff — to review any portion of the recorded ECG monitoring and trend reports detailing 24-hour heart rate and rhythm abnormalities.
“We offer the advantages of both a Holter and cardiac event monitor in one device to provide the most comprehensive long-term view of the heart rhythm,” said Jim Ott, chief technology officer, Biomedical Systems. “TruVue was created to help physicians accurately classify and assess atrial fibrillation and other complex arrhythmia. Our patented technology acquires and transmits every heartbeat for up to 30 days and immediately allows physicians access to information online —including both algorithm-triggered ECG-recordings and episodes recorded by patients.”
“Biomedical Systems has developed a revolutionary technology to assist in diagnosing and managing our atrial fibrillation patients,” said Carey S. Fredman, director, Electrophysiology Services, St. Luke’s Hospital, St. Louis, Mo. “TruVue gives me immediate access to review the onset and offset of sustained abnormal rhythms, giving me confidence to accurately diagnose my patients with AFib.”
In addition to Afib monitoring, pharmaceutical and medical device companies are also employing TruVue to assess the cardiovascular safety and efficacy associated with new drugs and devices. In pharmaceutical clinical trials, for example, TruVue’s heart monitoring capabilities support the efficient and accurate capture of valid data during Phase 1 to 4 clinical studies or in post-marketing surveys.
For more information: www.biomedsys.com


 July 22, 2025
July 22, 2025 



