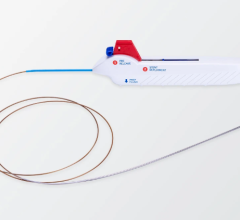
March 5, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech, today announced European CE mark approval of the VARIPULSE Platform for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AF) using pulsed field ablation (PFA). The VARIPULSE Platform is comprised of the VARIPULSE Catheter, a variable-loop multielectrode catheter; the TRUPULSE Generator, a multichannel PFA generator; and CARTO 3 System, the world’s leading 3D cardiac mapping system. The VARIPULSE Platform is the first and only CARTO -integrated PFA system, enabling an intuitive and reproducible workflow with real-time visualization and feedback mechanisms.
The safety and efficacy of the VARIPULSE Platform was investigated in the inspIRE trial, which included 186 patients in Canada and Europe.[ii] Updated one-year follow-up data was presented this month at the AF Symposium in Boston, demonstrating that among participants receiving optimal PFA applications, 80% achieved freedom from recurrence with zero primary adverse events.2** Furthermore, the primary effectiveness endpoint (PEE) of acute pulmonary vein isolation and 12-month freedom from atrial arrhythmia recurrence (AF, Atrial Tachycardia, or Atrial Flutter) was 75.6%.[iii] The study reported a low fluoroscopy time of 7.8 minutes, partly attributed to the integration of the VARIPULSE Catheter to the CARTO 3 System and a good safety profile with no (0.0%) primary adverse events reported.2
"CE mark approval of the VARIPULSE Platform represents a significant advance in catheter ablation technology, allowing electrophysiologists to offer patients in Europe pulsed field ablation treatment with real-time integrated 3D mapping,” said Tom De Potter,***MD, Associate Director, Cardiovascular Center, OLV Hospital Aalst, Belgium". “Significantly, the VARIPULSE Platform is fully integrated with the CARTO 3 System, enabling a simplified workflow with minimal fluoroscopy time. Most importantly, the recent published data on the VARIPULSE Platform demonstrates the safety using pulsed field ablation for patients being treated for AF."
Catheter ablation is a minimally invasive procedure performed by an electrophysiologist to treat heart rhythm disorders, including AF, by interrupting irregular electrical pathways in the heart by delivering either heat (radiofrequency ablation) or cold (cryoablation).[iv] PFA represents a new approach to treating AF, utilizing a controlled electric field to selectively ablate cardiac tissue that causes the irregular heartbeat through a process called irreversible electroporation (IRE).[v] Because the pulsed field energy is minimally thermal, IRE offers the potential to reduce the risk of damage to surrounding tissues including esophageal, pulmonary vein, and phrenic nerve injury.4
“At Biosense Webster, we continually seek to push the boundaries of science and technology innovation in cardiac ablation. CE mark approval of the VARIPULSE Platform is testament to this, now offering healthcare professionals the potential to improve outcomes for people living with atrial fibrillation while setting a new standard in cardiac electrophysiological mapping,” said Jasmina Brooks, President, Biosense Webster. “We believe pulsed field ablation has the potential to offer safer, more consistent and efficient workflows, and the VARIPULSE Platform uniquely offers physicians a simple and reproducible PFA workflow with 3D visualization, in real-time.”
AF is the most common type of cardiac arrhythmia, affecting over 11 million people in Europe.[vi],[vii],[viii] If left untreated, patients face a fivefold increased risk of stroke7, while their risk of death doubles7. By 2030, prevalence is projected to increase by up to 70 percent7 presenting an urgent need for innovative treatment solutions that deliver better outcomes for people living with AF while providing healthcare professionals with increased flexibility and efficiency.
For more information: www.biosensewebster.com
The VARIPULSE Platform is not available for sale in the United States.
*The TRUPULSE Generator received CE Mark in Europe in December 2023.
**Based on the final analysis of the InspIRE study focused on the pivotal phase (Wave II) per-protocol population of 186 patients with 12 months of follow up.
***Dr de Potter is a paid consultant to Biosense Webster Inc. He was not compensated for any media work.
References:
[i] [Insert link to CE marking approval notification].
[ii] ClinicalTrials.gov. A Study for Treatment of Paroxysmal Atrial Fibrillation (PAF) by Pulsed Field Ablation (PFA) System With Irreversible Electroporation (IRE) (inspIRE). Available at: https://clinicaltrials.gov/study/NCT04524364. Last accessed: January 2024.
[iii] Reddy,Vivek., et al. Paroxysmal AF Ablation Using a Variable-Loop Pulsed Field Ablation Catheter Integrated with a 3D Mapping System: One-Year Outcomes from inspIRE [abstract]. In: AF Symposium.; February 2–4; Boston
[iv] British Heart Foundation. Catheter Ablation. Available at: https://www.bhf.org.uk/informationsupport/heart-matters-magazine/medical/catheter-ablation. Last accessed: January 2024.
[v] Reddy VY, Neuzil P, Koruth JS, et al. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. JACC 2019;74(3)315-326.
[vi] Johan E.P. Waktare, MB, ChB, MRCP. Atrial Fibrillation. Circulation. 2002;106:14-16.
[vii] Velleca M, Costa G, Goldstein L, et al. A Review of the Burden of Atrial Fibrillation: Understanding the Impact of the New Millennium Epidemic across Europe. EMJ Cardiol. 2019; 7[1]:110-118.
[viii] Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S (2014) Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 6: 213-220.


 February 02, 2026
February 02, 2026