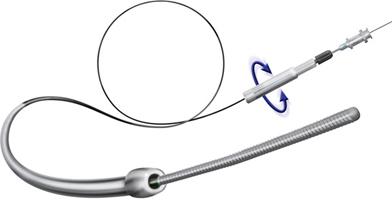
March 13, 2009 - BridgePoint Medical Inc. completed the first human clinical case in the U.S. using its CrossBoss CTO Catheter and Stingray CTO Re-Entry System.
The CrossBoss CTO Catheter and Stingray CTO Re-Entry System are under clinical investigation to demonstrate safety and effectiveness in the treatment of completely blocked arteries known as chronic total occlusions (CTO). BridgePoint Medical's clinical trial, Facilitated Antegrade Steering Technique for Chronic Total Occlusions (FAST-CTOs), was conditionally approved in January 2009 by the FDA.
The first clinical case was completed by William Lombardi, M.D., interventional cardiologist and cardiac catheterization lab director at North Cascade Cardiology in Bellingham, WA. The patient was a 61-year-old woman with a chronic total occlusion of her right coronary artery, chest pain (characterized as level three angina) and a positive stress test. The patient was referred to Dr. Lombardi for inclusion in BridgePoint Medical's FAST-CTOs clinical trial after a failed treatment attempt to cross the lesion by a referring physician.
Procedural completion took 35 minutes, resulting in the opening of the completely blocked vessel and return of normal blood flow to the patient's heart. "The BridgePoint Medical System allowed me to open this challenging total occlusion," Dr. Lombardi said. "BridgePoint Medical's clinical trial is designed to demonstrate the CrossBoss CTO Catheter and the Stingray Re-Entry System are broadly effective in re-establishing blood flow across completely blocked arteries and these devices will hopefully provide improved options for patient care."
The patient was released from the hospital the day after the procedure.
Annually 1.3 million worldwide patients suffer from chronic total occlusions of coronary arteries. Interventional cardiologists are currently unable to broadly treat patients with chronic total occlusions, which comprise approximately one third of all patients diagnosed with coronary artery disease. For these patients, a common alternative is open heart bypass surgery or palliative medical care.
For more information: www.bridgepointmedical.com


 October 28, 2025
October 28, 2025 









