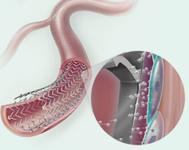
The Zilver 518 RX is a self-expanding nitinol stent.
October 21, 2009 – Cook Medical is introducing six new products in parallel to provide better treatment options to treat peripheral arterial disease (PAD), including new microwires, stents and angioplasty balloons that are integral to Cook’s advanced Leg Therapy Program.
Cook said the program offers the industry’s broadest line of products engineered specifically to address the dynamic circulation of the iliac, femoral, popliteal and infrapopliteal vascular systems. In addition, Cook will introduce a new thoracic aneurysm endograft and a sophisticated new delivery and retrieval system for Cook’s inferior vena cava filters for pulmonary embolism prevention to physicians attending VIVA 2009 in Las Vegas.
The company said its Approach CTO microwires, the first .014-inch wires designed specifically for crossing chronic total occlusions and extremely tight lesions in the peripheral arteries. The Advance line of balloon dilatation catheters comprises three low-profile balloons (14LP, 18LP, 35LP) that range in size and composition to treat lesions in the peripheral arteries (from the femoral through the popliteal and into the infrapopliteal region). Each balloon features a low crossing profile and small-sheath compatibility, which helps reduce the need for an invasive arterial entry and may shorten patient recovery time. Used as an adjunct to percutaneous transluminal angioplasty in the iliac, the Zilver 518 RX is a self-expanding nitinol stent with rapid exchange capabilities that ensure accuracy.
In addition to its new PAD products, at VIVA 2009 in booth 150, Cook also will have cutting-edge products on display to improve the prognosis of individuals with abdominal aortic aneurysm or pulmonary embolism. The Zenith TX2 Pro-Form endograft, engineered especially for procedures in which the product must be positioned in tight aortic arches, utilizes an improved delivery system that allows for carefully controlled deployment of the endograft to help establish proximal conformity of the device to the aortic wall. The new NavAlign IVC filter delivery system, introduced last month for both the Cook Celect and Günther Tulip filters, is designed to minimize trauma and streamline filter placement with easy jugular access.
For more information: www.cookmedical.com, www.bmcpromotion.com/Cook/100609/


 June 13, 2024
June 13, 2024 









