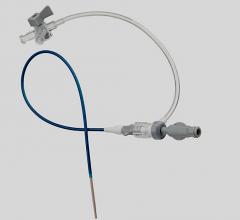
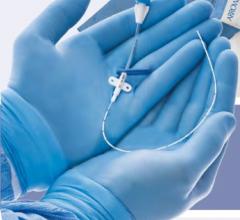
May 16, 2011 - Cordis announces the launch in Europe of Radial Solutions, a complete portfolio for transradial interventions. New to the portfolio is the Cordis Radialsource Transradial Access Kit. Radialsource Transradial Sheaths are designed to provide physicians with atraumatic and smooth access to the radial artery during diagnostic and interventional procedures.
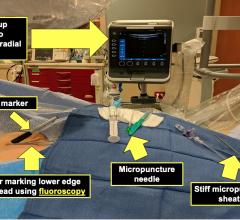
The rate of transradial procedures (TRA) in cardiovascular interventions is growing. Several studies have shown lower complication rates associated with transradial compared to the femoral approach. The "Radial Solutions" portfolio, including the Radialsource Transradial Sheaths, diagnostic and guiding catheters has been created in close cooperation with clinical partners to meet the requirements for this technique.
TRA offers significant benefits for patients, especially in patients who are at high risk of bleeding. Completing hemostasis after TRA is simpler, as the artery is superficial and easier to compress, increasing patient comfort.
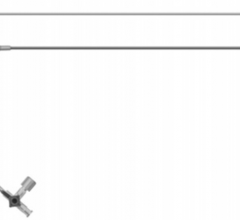
Cordis Radial Solutions portfolio comprises of Cordis' guiding and diagnostic catheters with new specific radial shapes, transradial sheath introducer, guide wires and professional education training.
The Radialsource Transradial Sheath incorporates a new tapering design in conjunction with a lubricious coating, offering the benefits of low insertion force with a smooth atraumatic entry, while enabling stability during procedure and ease of removal. Customers will be offered a choice of kit with a bare metal spring mini-guide wire or a polymer-coated mini-guide wire.
For more information: www.cordis.com

 June 10, 2020
June 10, 2020