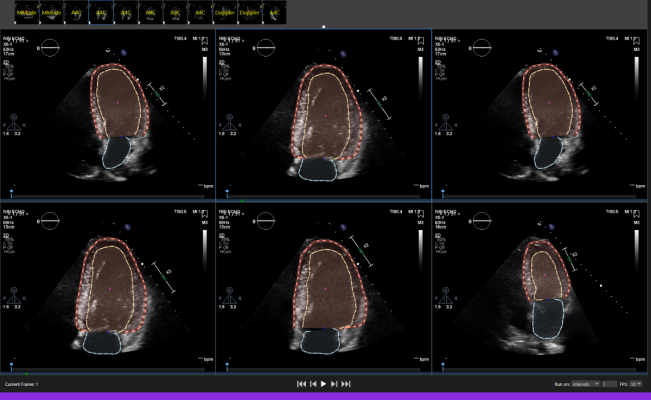
August 16, 2022 — Dyad Medical, Inc., the developer of the cloud-based AI technology for cardiac image analysis, announced that the U.S. Food and Drug Administration (FDA) has cleared its Echocardiogram application called Echo:Prio through the 510(k) pathway. Echo:Prio , part of the complete cardiac platform named Libby, offers fast, data-driven image analysis of echocardiogram images. It is an important decision-making support tool for index quantification of cardiac function saving the clinician time in diagnosis and treatment-decision making.
Echocardiograms are often the first step in diagnosing and developing a treatment plan for heart disease. The heart is the only organ in constant movement as it pumps blood throughout the body. When analyzing the left ventricle, any abnormal motion may be a sign of heart disease. Most imaging labs rely on visual detections of these abnormalities to diagnose and manage heart disease. This subjective process requires a complete view of the endocardium which is not always available. Furthermore, additional factors influencing image interpretations include operator skill level and variations in heart rates and cardiac loading, all of which may be addressed using automated analysis.
“The current process of manual echocardiographic assessment of myocardial function is time consuming and lacks consistency between readings. The FDA’s clearance of Dyad Medical’s Echo:Prio application allows us to provide operators and physicians an essential computer-assisted tool for echocardiographic analysis. Our solution is proven to be consistent and can be used across all systems, regardless of an operator’s skill level,” said Dr. Ronny Shalev, CEO and co-Founder of Dyad Medical, Inc.
Libby Echo:Prio is embedded in the current workflow of imaging operators, physicians, and researchers. The Libby platform is cloud-based, offering users a secure way to use it from any location on any device. The AI-powered platform provides users an immediately-available second opinion when diagnosing cardiac images saving them critical time that can be spent with patients.
“Dyad Medical’s Echo:Prio application allows physicians to prioritize patient treatments and deliver the life-saving care needed for those who need it most,” said Shalev. “Echocardiogram analysis done manually is time consuming and must be done consistently across all patients and operators. Echo:Prio is expanding the physician’s view so they have greater confidence in their diagnosis leading to greater patient satisfaction and retention.”
This is the second FDA clearance Dyad Medical has received for its Libby cardiac imaging analysis platform. Dyad Medical’s intravascular optical coherence tomography application is also FDA cleared. Focusing on AI-powered analysis of cardiac images, the company continues to enhance its applications for other imaging modalities.
For more information: www.dyadmedical.com


 January 28, 2026
January 28, 2026 









