January 3, 2014 — Endologix Inc., developer and marketer of treatments for aortic disorders, announced that it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin a clinical trial to evaluate the safety and effectiveness of the Nellix EndoVascular
Aneurysm Sealing System (EVAS) for the endovascular repair of infrarenal abdominal aortic aneurysms. The study, EVAS FORWARD-IDE, is one of several clinical studies that make up the broader EVAS FORWARD Clinical Program aimed at establishing clinical and economic evidence for EVAS using Nellix. The EVAS FORWARD-IDE study is approved to enroll 180 patients at up to 30 sites in the United States, Canada and Europe.
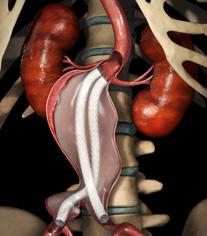
The Nellix EVAS system is a new generation of abdominal aortic aneurysm (AAA) therapy designed to seal the entire aneurysm with a biocompatible polymer. Nellix is the first and only EVAS product and was developed to simplify procedures, reduce re-interventions and expand the treatable patient population. Endologix received CE mark for the Nellix EVAS System in the first quarter of 2013 and the commercial release of the product in Europe is currently underway.
"The unique ability of Nellix to seal the entire aneurysm sac is breakthrough technology and is the first meaningful advancement in AAA repair since endovascular
grafts were introduced back in the late 1990s,” said Jeffrey Carpenter, M.D., chief of surgery and chairman, Cooper University Health Care, Camden, N.J., and the study’s national principal investigator. “We believe Nellix has the potential to dramatically change the way we treat AAA."
“Based on the anticipated enrollment timeline and one-year follow up period, Nellix could potentially be available to physicians and patients in the U.S. in the second half of 2016," said John McDermott, CEO and chairman, Endologix.
For more information: www.endologix.com