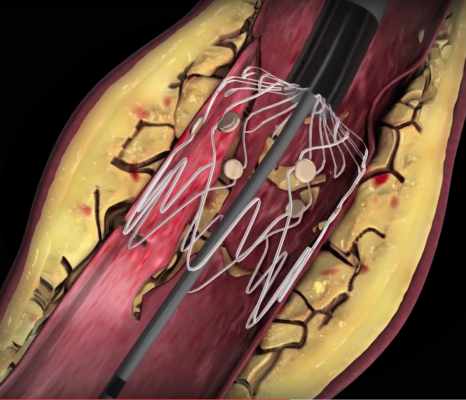
November 8, 2019 — Dissection is a frequent and clinically problematic outcome of percutaneous transluminal balloon angioplasty (PTA), and there is no current safe solution for revascularization of below-the-knee (BTK) arteries. The TOBA II BTK Trial looked at using small stents to “tack” the vessel if needed without using a full sized stent, which leads to stent fractures BTK.
The trial, presented as a late-breaker at the 2019 Vascular Interventional Advances (VIVA) annual meeting, met all endpoints in a 100 percent dissected vessel population. There are no FDA-approved implants for use BTK, which lead to this trial texting a new technology, the Intact Vascular Tack endovascular system (4 French). High rates of dissection resolution, wound improvement, and freedom from clinically driven target lesion revascularization (CD-TLR), support the Tack endovascular system as an ideal adjunct to PTA and potentially as the first permanent vascular implant to improve results of infrapopliteal angioplasty, said presenter George Adams, M.D., University of North Carolina Health Care.
TOBA II BTK was a prospective, multicenter, single-arm global pivotal study, is the first investigational device exemption clinical trial approved to investigate safety and effectiveness of a permanent implant to repair dissections in BTK arteries. The study evaluated the device in 233 patients who had ≥ 1 dissection requiring repair after PTA in the mid/distal popliteal, tibial and/peroneal arteries. There were 918 Tacks implanted from the mid popliteal to 1 cm above the tibiotalar joint, with 122 placed in the distal third of the vessels.
The Tack system has four preloaded self-expanding nitinol implants on a single catheter. Each implant is 6 mm long and self sizes to vessels ranging from 1.5 to 4.5 mm in diameter, using sufficient radial force to appose dissected tissue against the vessel wall. Primary safety and efficacy endpoints were analyzed at 6 months and compared with performance goals derived from the critical limb ischemia (CLI) literature.
TOBA II BTK met both primary endpoints (P < .0001) of safety (30-day major adverse limb event or perioperative death) and efficacy (6-month major adverse limb event or 30-day perioperative death). All (100 percent) dissections were resolved following Tack implantation, with 92 percent 6-month Kaplan-Meier freedom from CD-TLR, 87.3 percent 6-month Kaplan-Meier target lesion patency, and 95.7 percent 6-month Kaplan-Meier amputation-free survival. Wounds were healed or improved in 73.8 percent and Rutherford category was ≤ 3 at 6 months in 74 percent of CLI patients.
Find information on all the VIVA 2019 Late-breaking Clinical Trials

