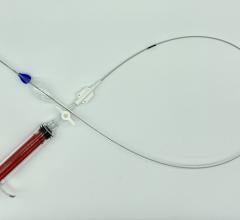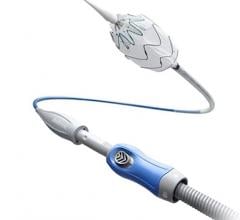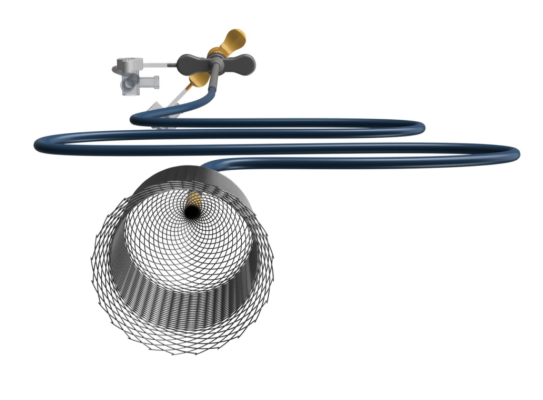
September 13, 2022 — The ŌNŌ endovascular retrieval system was recently used to remove a malpositioned leadless pacemaker (LPM) that embolized to the right pulmonary artery in a 52- year-old patient, ŌNŌCOR LLC announced. The team at UCLA Medical Center, Los Angeles, California, percutaneously removed the wayward device from the patient using a combination of ŌNŌ, commercially available endovascular snares and a large bore sheath.
“Our patient was referred in from a hospital outside of UCLA, where unfortunately, they experienced an embolization of the LPM device from the right ventricle into the right pulmonary artery, where it became entrapped in a small segmental branch,” said Dr. Zach Haber, a UCLA Interventional Radiologist. “The LPM, which looks like an AA battery with hooks on it, was noted to be out of position at a recent follow-up appointment. Removal was initially deemed too risky by the referring hospital, so it was left in the pulmonary artery for several weeks,” said Dr. Haber.
“Our team at UCLA reviewed the case and thought that using ŌNŌ, we could safely capture and remove the LPM from the pulmonary artery in an atraumatic fashion. Fortunately, that is exactly what we were able to accomplish,” said Dr Aron Bender, a UCLA Electrophysiologist.
“Leadless pacemakers convey many advantages over the older lead-based systems,” said Dr. Bender, the patient’s electrophysiologist at UCLA. “However, embolization and misplacement of such devices, though rare, is an unavoidable risk. It is incumbent upon us as clinicians to be able to manage this complication safely, and preferably without surgery,” said Dr. Bender.
“I think the ŌNŌ represents a significant advancement in bailout technology, and as we saw here today, ŌNŌ will be very helpful in helping us to avoid urgent/emergent surgery moving forward,” said Dr. Jamil Aboulhosn, head of the UCLA Adult Congenital heart disease program.
“I’d say that the game has just changed significantly thanks to ŌNŌ and ŌNŌCOR,” added Dr. Daniel Levi a Pediatric Cardiologist who assisted Dr Haber in the procedure.
The ŌNŌ is a novel device designed to receive, align, compress, and remove material (non-biologic and biologic) from the vascular system. ŌNŌ is intuitive to use and is compatible with commercially available vascular sheaths, endovascular snares and other graspers. ŌNŌ received FDA clearance in May 2022 and is available at select sites throughout the United States.*
“ŌNŌ was designed to add an extra layer of security to advanced endovascular procedures,” said Mark Piper, CEO of ŌNŌCOR. “Thanks to the team at UCLA, we can now clearly see that ŌNŌ’s reach extends into the world of electrophysiology.”
*The ŌNŌCOR LLC ŌNŌ retrieval device is indicated for use in the cardiovascular system to retrieve foreign objects using minimally invasive procedures. For complete instructions and other important safety information for ŌNŌ, please refer to the Instructions for Use.
For more information: www.onocorvascular.com
See also: ŌNŌCOR Receives FDA Clearance for Novel Endovascular Retrieval Technology


 September 18, 2025
September 18, 2025