January 2, 2018 — RenalGuard Solutions Inc. reported positive results from a first-in-man feasibility study focusing on a novel use of the RenalGuard System to manage fluids during diuretic therapy in congestive heart failure patients suffering from fluid overload. The results were recently presented at the annual Devices in Heart Failure (D-HF) Congress, Dec. 15-16 in Berlin, Germany, by Prof. Piotr Ponikowski, M.D., of the Wroclaw Medical University, Poland, and Prof. Felix Mahfoud, M.D., of the Saarland University Hospital, Homburg, Germany.
The presentation reviewed the ways in which current treatment strategies for hospitalized patients with heart failure remain inadequate. Annual hospitalizations for heart failure exceed 1 million in both the United States and Europe, and more than 90 percent are due to symptoms and signs of fluid overload. Recurrent fluid overload in heart failure patients has uniformly been associated with worse outcomes independent of age and renal function; 25 percent of hospitalized patients will be re-hospitalized within three months, with a one-year mortality rate of 26 percent.
The presenters also identified the drawbacks of diuretic therapy, the cornerstone therapy for fluid overload, which acts primarily by inducing fluid loss. An individual patient’s response to diuretic therapy is often variable and unpredictable. If the patient sees excessive urine output due to the diuretic, this rapid fluid loss can induce a condition termed “diuretic resistance,” which blunts the continued function of diuretics and may result in acute kidney injury.
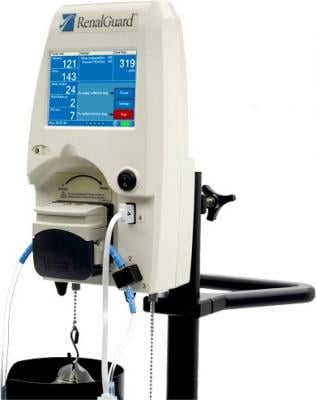
Originally developed for the treatment of contrast-induced acute kidney injury (CI-AKI), RenalGuard Therapy offers a potential solution to improve the impact of diuretic therapy in the treatment of fluid overload in heart failure patients. The results presented at D-HF followed the treatment of 10 diuretic resistant patients with heart failure symptoms receiving diuretic therapy while their fluid management was controlled by the RenalGuard System.
“None of the patients we treated experienced a fluid loss rate greater than the settings we established,” said Ponikowski, who also serves as the chairman of the European Society of Cardiology 2016 Heart Failure Guidelines Committee. “RenalGuard Therapy is remarkably simple and safe, and works automatically to carefully achieve and control the desired fluid balance.”
The RenalGuard System measures the patient’s urine output, then infuses a volume of saline to maintain the desired fluid balance. The clinician can set a maximum fluid loss rate, beyond which RenalGuard will not allow the patient’s fluid balance to drop, thus limiting the potential of excessive fluid loss. This may allow clinicians to increase the dose of diuretic without increasing the risk of diuretic resistance.
“There is a clear unmet clinical need for alternative methods of fluid removal with superior efficacy in patients with heart failure. This first-in-man study demonstrated that RenalGuard can safely be used in these patients while maintaining the proper conditions to both prevent diuretic resistance, and increase the removal of excess fluid from the patient,” said Mahfoud. “Our initial experience with the RenalGuard System in heart failure patients is very promising, and we look forward to advancing our understanding of the benefits of this therapy to patients at risk.”
The RenalGuard System is CE-marked and commercially available in Europe. A pivotal study is underway in the United States to support a planned premarket approval (PMA) filing with the U.S. Food and Drug Administration (FDA) in 2018 for the prevention of CI-AKI.
For more information: www.renalguard.com


 January 05, 2026
January 05, 2026 









