September 30, 2013 — At RSNA 2013, GE Healthcare will highlight the latest hardware and advanced applications for its Discovery IGS angiography platform. These updates are geared towards addressing the challenges of healthcare providers, support clinical decision making and helping clinicians in refining overall patient care.
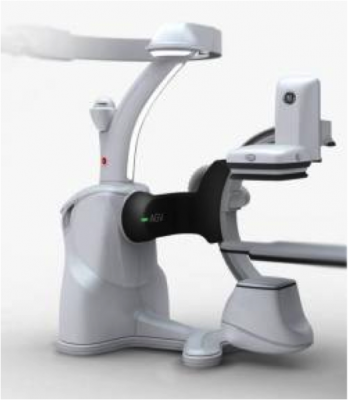
The Discovery IGS 730 is the first interventional X-ray system designed to capture the advantages of both floor- and ceiling- mounted systems. It offers laser-guided motion technology on a wireless, tether-free, motorized mobile gantry for predictable and precise trajectories, Wide Bore 3-D for ease in 3-D acquisitions and more than 20 advanced applications available.
The Discovery IGS 730 C-arm is mounted on an advanced guided vehicle (AGV), a motorized and fully mobile system. Based on laser guidance, the AGV can move freely from imaging position to parking or back-out positions, using predefined trajectories to provide total patient access. The Discovery IGS 730 features “One-Touch-Back-Out,” enabling fast and easy gantry movement away from the patient. The motion is predictable, precise and easy to use, allowing fine control and positioning at any moment in the procedure. Parking locations and back-out distances are customizable for different room configurations.
For more information: www.gehealthcare.com


 October 24, 2025
October 24, 2025 









