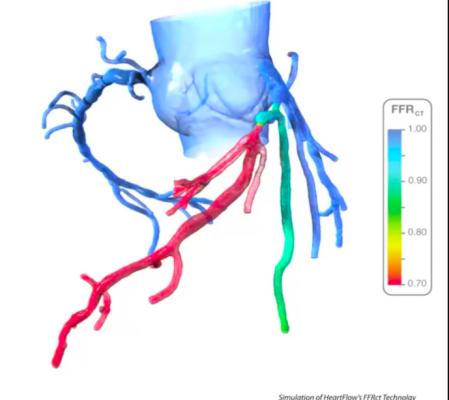
An example of HeartFlow's FFR-CT assessment, which shows areas of low blood flow based on computation fluid dynamics from a CT scan of the heart. The vendor's new PreRead software will use AI to clearly and reproducibly identify areas of ischemia due to coronary artery occlusion and act as a second set of eyes for emergency rooms evlautaing chest pain patients.
December 13, 2021 - HeartFlow Inc., the leader in revolutionizing precision heart care, today announced it has submitted a 510(k) premarket application to the U.S. Food and Drug Administration (FDA) to add advanced anatomic assessment and plaque evaluation to the HeartFlow fractional flow reserve-computed tomography (FFR-CT) analysis. The PreRead anatomic assessment will help identify the presence and location of narrowings or stenoses in the coronary arteries based on coronary computed tomography (CT) scans.
The HeartFlow Plaque technology will provide plaque volume and characterize the type of plaque present. By adding the anatomic assessment and plaque evaluation to the physiological information currently provided by the HeartFlow Analysis, physicians will gain a more comprehensive understanding of a patient’s coronary disease burden and support efficient risk stratification of patients who may be at high risk of death from a heart attack.
“HeartFlow is committed to becoming an indispensable partner to physicians in delivering precision heart care by providing a broad portfolio of game-changing innovations that leverages our core deep learning technology,” said John H. Stevens, M.D., president, CEO and co-founder of HeartFlow. “The FDA submission is an important step towards delivering a solution that we believe supports complete cardiovascular care by providing clinicians with advanced insights about anatomy, physiology and plaque for their patients with coronary artery disease.”
PreRead is designed to provide a rich anatomic assessment of each coronary CT that, when considered by physicians in conjunction with other patient clinical information, helps physicians to quickly identify where coronary disease is present and understand the severity of the narrowing. The PreRead assessment highlights areas of modeled stenosis greater than 30% for vessels 1.8 mm or larger in diameter. This information will be provided as a 2D image of the coronary arteries, with areas of concern color coded by severity. In addition, CT images of each of the three main arteries are provided so physicians can more easily confirm the PreRead color-coded narrowings.
The PreRead assessment is intended to support clinical workflow efficiency, diagnostic accuracy and CT reader consistency as measured by repeatability and reproducibility. In an internal study, the PreRead assessment was 35% more repeatable and 47% more reproducible in classifying percent stenosis than expert CT readers’ (Level 3) classification of the same cases.[1]
In addition, the HeartFlow Plaque feature is based on a fully automated deep-learning (a form of AI) algorithm for characterizing and quantifying plaque. In an internal study, the HeartFlow Plaque technology was found to be more reliable than expert CT readers in identifying different types of plaque and quantifying total plaque volume.[2]
About the HeartFlow FFR-CT Analysis
Starting with a standard coronary CT scan, the HeartFlow Analysis leverages algorithms trained using deep learning and highly trained analysts to create a digital, personalized 3D model of the heart. The HeartFlow Analysis then uses powerful computer algorithms to solve millions of complex equations to simulate blood flow and provides FFR-CT values along the coronary arteries. This information is used by physicians in evaluating the impact a blockage may be having on blood flow and to determine the optimal course of treatment for each patient. A positive FFR-CT value (≤0.80) indicates that a coronary blockage is impeding blood flow to the heart muscle to a degree which may warrant invasive management.
Data demonstrating the safety, efficacy and cost-effectiveness of the HeartFlow Analysis have been published in more than 500 peer-reviewed publications, including long-term data out to five years. The HeartFlow Analysis offers the highest diagnostic performance available from a noninvasive test.[3] To date, clinicians around the world have used the HeartFlow Analysis for more than 100,000 patients to aid in the diagnosis of heart disease.
About HeartFlow
HeartFlow is the leader in revolutionizing precision heart care, uniquely combining human ingenuity with advanced technology. HeartFlow’s non-invasive HeartFlow FFR-CT Analysis leverages artificial intelligence to create a personalized three-dimensional model of the heart. Clinicians can use this model to evaluate the impact a blockage has on blood flow and determine the best treatment for patients. HeartFlow’s technology is reflective of our Silicon Valley roots and incorporates over two decades of scientific evidence with the latest advances in artificial intelligence. The HeartFlow FFRct Analysis is commercially available in the United States, U.K., Canada, Europe and Japan.
For more information: www.heartflow.com
References:
1. HeartFlow Internal Study. Results based on Modified CADRADs. December 2021
2. HeartFlow Internal Study. December 2021
3. Driessen, R., et al. J Am Coll Cardiol. 2019;73(2),161-73.


 November 14, 2025
November 14, 2025 









