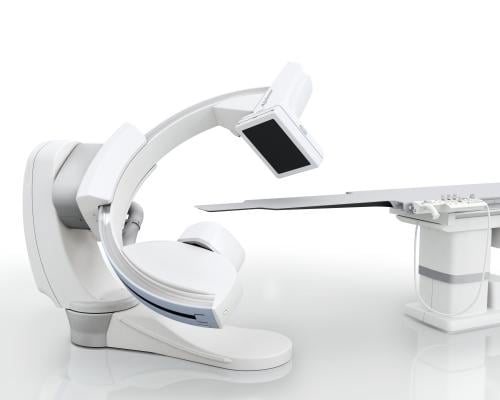
August 21, 2019 — Jackson Memorial Hospital in Miami celebrated the opening of two newly renovated cardiac catheterization suites during a ribbon-cutting ceremony on August 19, 2019. The suites feature the new Alphenix Core+ from Canon Medical Systems, which provides diverse imaging without compromising image quality or performance. Jackson Memorial is the first hospital in Florida offering this new technology.
“Almost six years ago, when Miami-Dade voters approved the bond, we promised a huge makeover for Jackson – new facilities, renovated patients units and new technology,” Carlos A. Migoya, president and CEO of Jackson Health System, said at the ribbon cutting. “We are so excited to launch these new catheterization labs, which offer the most sophisticated and advanced technology to our cardiology patients.”
Made with what Canon calls the world’s first high-definition detector for resolving fine details, the hybrid 12 x 12-inch panel on the Alphenix Core+ is combined with high-definition flat panel technology.
The Alphenix Hi-Def Detector technology helps clinicians see finer details during complex interventional procedures, such as stent positioning and stent apposition, wire and catheter navigation through the stent struts, and observation of coil deployment. The slim, off-center C-arm design on the Alphenix Core+ also allows steep angulations for optimized vessel profiling during cardiac interventions. In addition to offering high-quality imaging, the system has several components that allow for a wider range of procedures.
This state-of-the-art system, combined with the expertise of Jackson physicians, will provide patients with the highest quality experience and best outcomes, said Alexandre Ferreira, M.D., chief of cardiology and medical director of cardiology at Jackson Health System.
“We are so proud to unveil the new cardiac catheterization labs, which will be the primary treatment area for many diagnostics and interventional heart procedures,” said Ferreira. “With the use of the Alphenix Core+, patients will be able to receive a wider range of procedures without needing to be moved, while the care team access controls with a digital tablet. The rooms also offer 3-D imagery on live displays, and the lowest possible radiation doses.”
With this new technology, Jackson’s cardiology team can track in real time the estimated skin dose of X-ray exposure, which is displayed on the screen with designated colors ranging from lowest to the highest exposure. This is a vital patient safety feature.
Patients in need of procedures, such as angioplasty, stent implantation and heart catheterization, will be treated in these new cath labs. These minimally invasive tests and procedures can be performed instead of heart surgery to access heart and blood vessels.
Earlier this year, Jackson launched the Heart Surgery Institute, which combined a well-known group of cardiac surgeons with its existing cardiology team, providing the most comprehensive cardiac services in the region for patients of all ages.
For more information: www.jacksonhealth.org


 October 24, 2025
October 24, 2025 









