January 12, 2023 — Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received conditional FDA Investigational Device Exemption (IDE) approval to initiate its pivotal clinical trial for the treatment of coronary de novo lesions.
This comes less than eight months after the company received its first IDE approval for SELUTION SLR in the treatment of below-the-knee (BTK) indications; as well as occlusive disease of the superficial femoral artery (SFA); and coronary In-Stent Restenosis (ISR).
Enrollment of the Selution SLR coronary de novo study will begin in the US within the next few months.
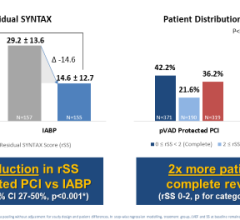
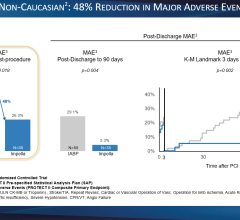
This will complement the substantial experience that the company has already gained with the Selution DeNOVO trial in Europe (ClinicalTrials.gov Identifier: NCT04859985). More than 800 patients of the 3,326 planned have been enrolled in this ground-breaking coronary randomized controlled study comparing Selution SLR vs. any limus drug-eluting stent (DES). The study is powered to demonstrate superiority of Selution SLR drug-eluting balloon (DEB) over DES in coronary de novo artery disease. This is the largest DEB study ever initiated and has the potential to change medical practice where implants (metal stents) have been the standard of care for more than 30 years.
"Treatment of de novo coronary arteries with drug-eluting balloons is a breakthrough in revascularization of coronary artery disease. The Selution SLR coronary de novo study is the first of its kind in the USA and will provide important data on the efficacy and safety of sirolimus- eluting balloon as a viable alternative to drug-eluting stent, leaving nothing behind post-PCI and eliminating in-stent restenosis and related complications" said Dr. Ron Waksman, Professor of Cardiology at Georgetown University, Director of Cardiovascular Research at MedStar Heart and Vascular Institute, Washington DC and Chairman of the MedAlliance Coronary Study Steering Committee.
“Coronary de novo lesions are the largest potential opportunity for use of DEB’S: the data has shown clearly that DES don’t work well in small vessels, long, or bi-furcated lesions or in patients with diabetes or risk of high bleeding complications. These patients represent 60% of all patients currently treated with DES, who may now benefit from this exciting new DEB technology” added Jeffrey B. Jump, Chairman and CEO of MedAlliance.
Selution SLR was awarded CE Mark Approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary artery disease in May 2020.
MedAlliance’s unique DEB technology involves MicroReservoirs which contain a mixture of biodegradable polymer intermixed with the anti-restenotic drug sirolimus applied as a coating on the surface of an angioplasty balloon. These MicroReservoirs provide controlled and sustained release of the drug for up to 90 days.
Selution SLR 014 PTCA is commercially available in Europe, Asia, the Middle East and the Americas (outside USA) and most other countries where the CE Mark is recognized. Over 10,000 coronary units have already been used for patient treatment in routine clinical practice or as part of clinical trials.
For more information: https://medalliance.com/
Related content:
First US Patient Enrolled in SELUTION SLR IDE Peripheral Study

 August 14, 2023
August 14, 2023