November 11, 2015 — Medtronic plc announced that the Medtronic Micra Transcatheter Pacing System (TPS) was successfully implanted in nearly all patients in the trial and met its safety and effectiveness endpoints with wide margins. These data, from the Medtronic Micra TPS Global Clinical Trial, were presented during a late-breaking special report session at the 2015 American Heart Association Scientific Sessions and simultaneously published in the New England Journal of Medicine.
"We are extremely pleased with the remarkably successful implant rates and safety profile of the Micra pacemaker, including the absence of device dislodgments. We are especially confident in these results because the trial included patients with serious comorbidities from 19 countries on five continents around the world," said Dwight Reynolds, M.D., trial principal investigator and regent's professor and chief of the Cardiovascular Section at the University of Oklahoma Health Sciences Center. "The Micra TPS not only met its trial endpoints, but also provided a significant reduction in healthcare utilization due to fewer major complications compared to conventional pacing systems, which is particularly important in an era of value-based healthcare."
In the Micra trial, 96 percent of patients (700 of 725; six-month Kaplan-Meier estimate) experienced no major complications, which is 51 percent fewer major complications than seen in patients with conventional pacing systems (hazard ratio: 0.49; 95 percent CI, 0.33 to 0.75; P=0.001). Major complications included cardiac injuries (1.6 percent), complications at the groin site (0.7 percent) and pacing issues (0.3 percent). Notably, there were no dislodgments, no systemic infections and very few (0.4 percent) system revisions (meaning extraction, repositioning or replacement). These low complication rates were achieved despite the inclusion of high-risk patients worldwide, including patients with chronic obstructive pulmonary disease (COPD).
The presentation included a comparison of Micra TPS safety performance to a pre-defined, historical control group consisting of more than 2,500 patients from six studies of commercially available, conventional pacing systems. Compared to patients with conventional systems, the patients in the Micra trial were older and had more comorbidities, yet had fewer major complications.
Almost all patients in the trial, 98.3 percent (292 of 297) had low and stable pacing thresholds at six months, yielding projected average longevity for the device of more than 12 years (300 patients at six months).
In addition, the low major complication rates experienced by Micra patients resulted in significant reductions in healthcare utilization compared to conventional pacing systems: Micra patients had 54 percent fewer hospitalizations (p=0.011) and 87 percent fewer system revisions (p<0.001) than observed in the historical control group.
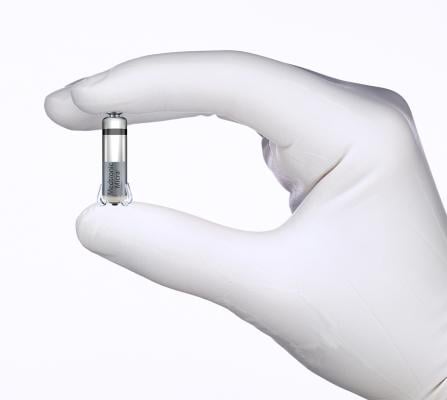
At less than one-tenth the size of traditional pacemakers, the Micra TPS is the world's smallest pacemaker. It is cosmetically invisible and small enough to be delivered with minimally invasive techniques through a catheter, and implanted directly into the heart.
Comparable in size to a large vitamin, the Micra TPS does not require the use of leads to deliver pacing therapy; rather, its flexible tines attach to the interior of the right ventricle. The tines can be disengaged during the implant process without causing trauma to the cardiac tissue, allowing the device to be repositioned during implant and retrieved if needed. Micra TPS also is the first transcatheter pacing system to be awarded European CE Mark for 1.5T and 3T full body magnetic resonance imaging (MRI) scanning, providing patients with access to the most advanced imaging diagnostic procedures.
In the United States, the Micra TPS is an investigational device and not yet approved for commercial use. The device was awarded CE Mark in April 2015 based on earlier data from the Medtronic Micra TPS Global Clinical Trial.
The trial enrolled 744 patients; it is ongoing and will continue to evaluate the safety and efficacy of the device through a single-arm, multi-center study at 56 centers in 19 countries. Primary endpoints of the trial were freedom from device-related or procedure-related major complications with target performance of >90 percent (lower CI >83 percent) at six months, and low and stable pacing thresholds as demonstrated by <= 2V and no increase of >1.5V (relative to implant) and target performance of >89 percent (lower CI >80 percent) in the first 300 patients at six months.
For more information: www.medtronic.com


 January 29, 2026
January 29, 2026 









