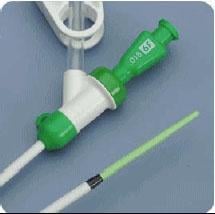
February 8, 2011 – The U.S. Food and Drug Administration (FDA) said Merit Medical Systems initiated a Class I recall for one lot of its Prelude Short Sheath Introducer, because the tips may detach during use.
The company said a detached tip may cause arterial injury, hemorrhaging or other serious events, or may enter into the bloodstream, causing blood clots.
The recall is specific to 378 devices. It includes the Prelude Short Sheath Introducer, 7 French-SMT, 4 cm, reference number PSS-7F-4-038MT, Sterile EO, Merit, lot/serial number H179575.
The company notified sales representatives and authorized distributors on Dec. 17, 2010, by email, phone and fax to immediately contact customers to identify, quarantine and return all unused inventory.
For more information, call Merit Medical Systems at 801.316.4932 or 800.356.3748 between 8 a.m. and 5 p.m. Monday through Friday (MST).
Healthcare professionals and consumers may report adverse reactions or quality problems they experience using these products to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
For more information: www.fda.gov/Safety/MedWatch/default.htm

 June 10, 2020
June 10, 2020