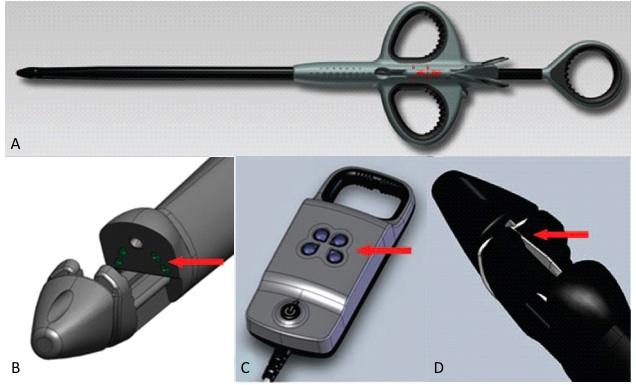
The NeoChord DS1000 (A) has the following features: A tip with expandable jaws to grasp the leaflet and four fiber-optic channels (B), each of which corresponds to an indicator light on the device monitor (C) to confirm proper leaflet capture. The needle (D) is also included in the device to deploy the suture after confirmation of leaflet capture. Image courtesy of NeoChord Inc.
March 29, 2016 — NeoChord Inc. announced that David H. Adams, M.D., and Michael A. Borger, M.D., Ph.D., will serve as national co-principal investigators of the company’s U.S. pivotal trial scheduled to commence later this year. The trial will study the NeoChord DS1000 system for minimally invasive repair of degenerative mitral valve regurgitation (DMR) without the use of cardiopulmonary bypass.
Adams is the Marie-Josée and Henry R. Kravis Professor and Chairman of the Department of Cardiovascular Surgery in the Icahn School of Medicine at Mount Sinai, and cardiac surgeon-in-chief of the Mount Sinai Health System. An acclaimed speaker, author, and renowned educator in the field of mitral valve reconstruction, Adams has given more than 350 invited lectures, authored more than 250 publications, and performed mitral valve surgery throughout the world. Over the past several years he has served as the national co-principal investigator of the Medtronic CoreValve U.S. Pivotal Trial. He is also a co-author with Prof. Alain Carpentier of the world’s widest selling textbook in mitral valve reconstruction.
Borger is professor of cardiothoracic surgery at Columbia Presbyterian School of Medicine, and the director of aortic surgery at the Cardiovascular Institute in the New York-Presbyterian/ Columbia University Medical Center. Previously, he served as professor and the associate director of the Leipzig Heart Center, the largest heart valve center in Europe. Borger is an internationally recognized leader in the fields of minimally invasive cardiac surgery, aortic surgery and novel surgical approaches to treat valvular heart disease. On the forefront of pioneering strategies to advance cardiac surgery throughout his career, he has published 250 contributions on a wide range of topics and served in numerous societal and academic journal leadership positions in both Europe and North America.
“To date, the NeoChord technology has demonstrated excellent outcomes from its commercial experience in Europe,” stated Adams. “I look forward to partnering with Dr. Borger as we initiate the U.S. pivotal trial for this cutting-edge technology.”
NeoChord received CE market clearance for the DS1000 system in December 2012. The device is not available for use in the United States.
For more information: www.neochord.com


 July 31, 2024
July 31, 2024 









