Image courtesy of University of Missouri School of Medicine
October 24, 2018 — For those living with diabetes, monitoring blood glucose accurately is necessary to prevent diabetes-related complications such as heart attacks, blindness and coma. Researchers from the University of Missouri School of Medicine and the Massachusetts Institute of Technology (MIT) recently evaluated the accuracy of an MIT-developed technology to monitor blood glucose levels without needles or a finger prick. Early results show the noninvasive technology measures blood glucose levels as effectively as a finger-prick test — without drawing blood.
The study, “Evaluation of accuracy dependence of Raman spectroscopic models on the ratio of calibration and validation points for non-invasive glucose sensing,” measured the blood glucose levels of 20 healthy, non-diabetic adults prior to drinking a glucose-rich beverage. Blood glucose levels were then measured in intervals over the next 160 minutes using three methods: spectroscopy, IV blood test and finger prick. The tests are designed to determine how much glucose remains in the blood and if a patient’s insulin-regulating mechanisms are working effectively. The researchers found that spectroscopy predicted glucose values as accurately as a finger-prick test.
The study was recently published online by Analytical and Bioanalytical Chemistry.1
“Currently, blood glucose levels are tested through a finger prick or intravenously. The approach we studied is noninvasive and uses a laser to monitor glucose levels in the skin,” said Anandhi Upendran, Ph.D., director of biomedical innovations at the MU School of Medicine Institute for Clinical and Translational Science and co-author of the study. “With diabetes on the rise, the development of an accurate, efficient and inexpensive alternative method to test blood glucose levels is an urgent clinical need.”
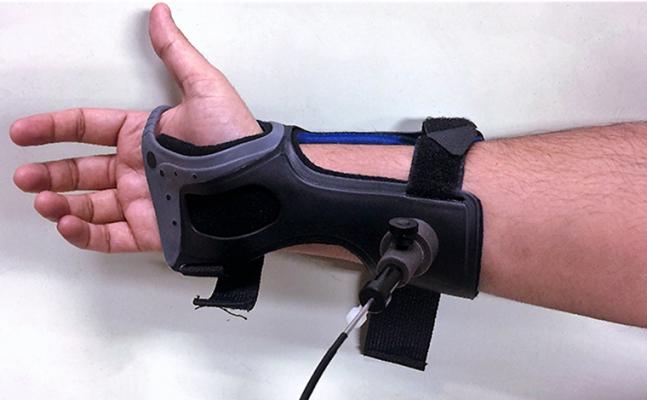
Developed by researchers from MIT, the device uses a technique called Raman spectroscopy to measure the chemical composition of skin and extract the amount of glucose out of other skin compartments. A fiberoptic cable attached to a wristband passes laser light onto the skin to detect different components in the skin, such as fat tissue, protein, collagen and glucose molecules. The shifts in wavelengths associated with glucose present in the blood creates a sort of molecular fingerprint that can be used to determine glucose levels.
“This is a technology that we have been pioneering for more than 20 years,” said Jeon Woong Kang, Ph.D., research scientist with MIT’s Laser Biomedical Research Center and co-author of the study. “We know that handheld skin-prick tests are not always accurate and may be uncomfortable for patients. The gold standard is intravenous blood testing, but frequent blood draws may not be an option for many patients. We were pleased to find that our initial results show Raman spectroscopy can measure glucose levels that are comparable to the finger stick devices. We hope that we can refine this method to be a noninvasive continuous glucose monitoring sensor.”
With more testing, the researchers hope spectroscopy can become an alternative method to test glucose levels in patients in clinical care settings who are not capable of frequent blood draws and, one day, in other settings as the technology becomes smaller and more portable. Future studies will examine the accuracy of the technology in patients with diabetes.
In addition to Upendran and Kang, authors include Surya Singh, Luis Galindo, Peter T. C. So and Ramachandra Rao Dasari, all with MIT; and Soumavo Mukherjee, Uzma Khan and Raghuraman Kannan, all with the MU School of Medicine.
Primary funding for the study is from the National Institutes of Health (P41-EB015871-30), the Samsung Advanced Institute of Technology and the Office of Medical Research at the MU School of Medicine. The study authors declare that they have no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
For more information: www.springer.com
Reference


 October 09, 2019
October 09, 2019