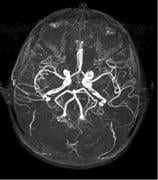
October 14, 2010 – An injectable magnetic resonance angiography (MRA) blood pool imaging agent has been released in Canada. Ablavar, which is made by Lantheus Medical Imaging and previously marketed as Vasovist, is indicated for contrast-enhanced MRA for visualization of abdominal or limb vessels in patients with suspected or known vascular disease.
“Ablavar is a welcome addition to the tools we currently have available in Canada for the evaluation of vascular disease, as it is specifically designed for use with MRA and allows for both first-pass imaging as well as steady-state imaging, which can provide additional information of the vasculature,” said Josephine Pressacco, M.D., Ph.D., Montreal Heart Institute. “The albumin-binding properties of this product allow us to obtain bright, high resolution images with a single low dose, which may reduce the need for additional or repeat testing for patients.”
Blood pool agents remain in the vasculature for an extended period of time, increasing the brightness of blood in a magnetic resonance diagnostic procedure, resulting in high-resolution images. The albumin-binding properties of Ablavar help it provide an expanded imaging window, making it possible to evaluate not only the location of disease, but also its extent and severity.
Magnetic resonance angiography (MRA) is a specific type of magnetic resonance imaging (MRI) procedure that provides pictures of blood vessels. MRA can show the blockage of the flow of blood to areas of the body such as the brain, kidneys and legs.
For more information: www.ablavar.com


 August 17, 2023
August 17, 2023 








