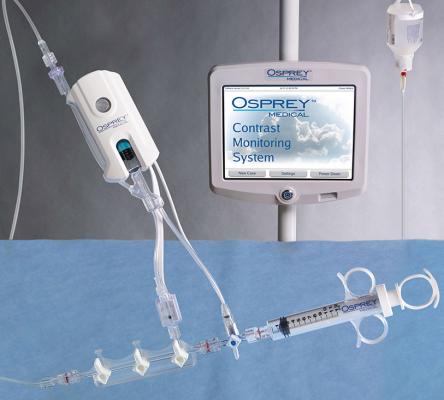
May 9, 2019 — Osprey Medical announced the launch of DyeMINISH, a global patient registry to evaluate the ongoing safety and performance of the DyeVert Contrast Reduction System during standard clinical use in a real-world patient population. The DyeMINISH registry is a retrospective, large-scale, multi-center study, with the first patient included on May 7, 2019. The registry is designed to enroll up to 10,000 participants and is expected to complete in late 2023.
The first patient was included in the DyeMINISH registry at St. Elizabeth Healthcare in Edgewood, Ky., by Mark Jordan, M.D., an interventional cardiologist. St. Elizabeth Healthcare operates five facilities throughout Northern Kentucky and more than 115 primary care and specialty office locations in Kentucky, Indiana and Ohio.
Jordan commented, “We are excited to be participating in the DyeMINISH Registry to help us evaluate our contrast-induced acute kidney injury (CI-AKI) prevention protocol. Reducing the risk of kidney damage during invasive heart procedures is of the highest priority for St. Elizabeth Heart and Vascular Institute, and is one of the many areas in which we seek to achieve the highest quality care. The multifaceted prevention protocol involves individualized assessment of patient’s risk of kidney injury, detailed preparation of the patient for the procedure, and efforts to limit exposure to imaging contrast volume. The protocol includes the use of the DyeVert System, a unique device that assists in reducing the volume of potentially harmful contrast agents delivered to patients during the procedure. The registry will provide the framework and analytics we need to demonstrate improvements in patient care in our cath lab.”
The DyeMINISH Registry includes two study cohorts. The Core Study cohort is comprised of patients who underwent coronary or peripheral angiography imaging procedure with the DyeVert System. The Comparative Health Outcomes Sub-Study includes patients undergoing the same procedures without DyeVert that can be matched to a DyeVert Group patient via propensity score matching.
The Core Study aims to evaluate the use of imaging dye thresholds, actual imaging dye delivered to the patient and imaging dye saved. The Core Study also aims to evaluate the incidence of adverse events, such as the incidence of CI-AKI, the relationship between imaging dye and CI-AKI, and other variables through to 120 days post-procedure. The Comparative Health Outcomes Sub-Study will compare the difference in health outcomes between those patients who received DyeVert versus the no DyeVert control via measures that include kidney function, imaging dye use, and incidence of major cardiac and renal adverse events.
For more information: www.ospreymed.com
Related Contrast Media Content
VIDEO: How to Avoid Acute Kidney Injury in the Cath Lab
VIDEO: Strategies to Avoid Acute Kidney Injury Caused by Cath Lab Contrast


 January 05, 2026
January 05, 2026 









