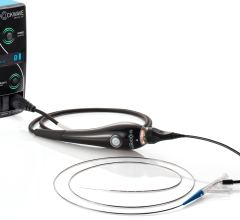
The Philips Healthcare OmniWire is the world’s first solid core fractional flow reserve (FFR) pressure wire for coronary artery interventional procedures.
August 18, 2020 — Philips Healthcare introduced its new OmniWire, the world’s first solid core fractional flow reserve (FFR) pressure wire for coronary artery interventional procedures. With its solid core construction, physicians can more easily maneuver the wire in the patient’s circulatory system to measure blood pressure along the vessel and guide the delivery of catheters and stents.
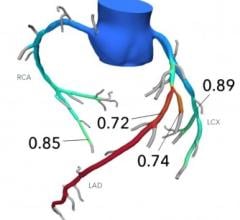
The new wire supports FFR and iFR (instant wave-free ratio) measurements, the only resting index supported by randomized controlled outcome trials.[1-3] It also integrates with the Philips IntraSight interventional applications platform, which can co-register iFR data onto the angiogram to precisely identify the parts of vessels requiring treatment.
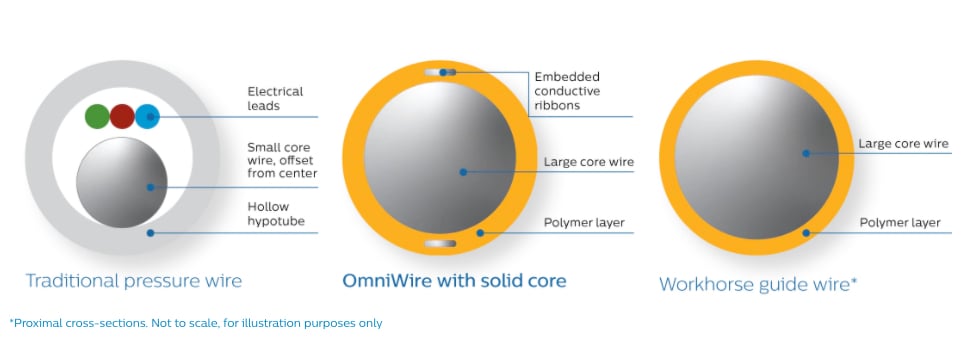
Traditional pressure wires use a hollow metal tube (hypotube) to house the wiring that transmits the pressure information. Due to their thin walls, these wires can be challenging to maneuver and can sometimes kink, potentially becoming damaged during the procedure. OmniWire is the world’s first solid core pressure guidewire, using advanced conductive ribbons embedded in its outer polymer layer to communicate pressure information. The distal part of the wire is made from Nitinol, a super-elastic, durable material that is commonly used in non-diagnostic, interventional ‘workhorse’ guide wires. The proximal part of the wire is constructed from a high-strength cobalt alloy that provides the high durability required for complex and multi-vessel cases.
“I have been very impressed with the handling of OmniWire, the new solid core design performed beautifully, and I was able to navigate the difficult case easily,” said Jasvindar Singh, M.D., director of the catheterization lab at Barnes Jewish Hospital and associate professor at Washington University in St. Louis, who performed the first human case with OmniWire in the country. “We used iFR co-registration and found that the patient needed a stent. I was then able to perform the whole procedure working over OmniWire. This is truly an innovation in percutaneous coronary interventions.”
The new wire supports both iFR and FFR indices. iFR continues to be adopted into clinical practice and has been validated in clinical outcomes studies with data from over 4,500 patients [1,2] as well as being recognized by the European Society of Cardiology (ESC), the Society for Cardiovascular Angiography and Interventions (SCAI), and the American College of Cardiology (ACC).[4-6]
OmniWire integrates with IntraSight, Philips’ secure interventional applications platform that integrates a comprehensive suite of clinically proven modalities including iFR, FFR, IVUS (intravascular ultrasound) and co-registration to simplify complex interventions and speed routine procedures.[7] With iFR pullback and co-registration, physicians can identify the precise locations causing ischemia, plan stent length and placement with a virtual stent, and predict physiologic improvement.
“With integration and co-registration on our IntraSight platform, measurement with iFR, and now enhanced wire performance thanks to OmniWire, we’re providing clinicians with an advanced solution at every step of the procedure,” said Chris Landon, senior vice president and general manager image guided therapy devices, Philips. “Physicians can confidently use a functional guidance strategy across all their patients, including in complex and multi-vessel cases. Today’s announcement demonstrates how our unique portfolio of systems, smart devices, software and services combines to deliver advanced procedure-oriented solutions.”
OmniWire is now available in the U.S. and Japan and has received clearance from the U.S. Food and Drug Administration (FDA) and approval from the Japan Pharmaceuticals and Medical Devices Agency.
For more information: philips.com/OmniWire
References:
6. ACC-SCAI recommendation letter for Volcano iFR CPT coding (93571, 93572).
7. Co-registration tools available within IntraSight 7 configuration via SyncVision.


 December 20, 2023
December 20, 2023