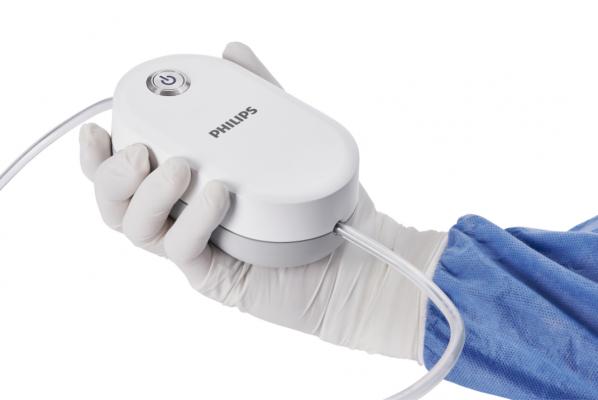
Philips introduced the QuickClear Mechanical Thrombectomy System. It is a simple thrombectomy solution provides physicians with an all-in-one, single-use aspiration catheter and pump system.
September 25, 2020 — Philips Healthcare launched its QuickClear mechanical thrombectomy system. The compact, single-use system provides an all-in-one aspiration pump and catheter for the removal of blood clots from the vessels of the peripheral arterial and venous systems. The system’s innovative all-in-one design is simple to use while eliminating the need for capital equipment or costly accessories, with easy setup supporting faster procedures times. The Philips QuickClear Mechanical Thrombectomy System has received U.S. FDA 510(k) clearance and is available for sale in the U.S.
“QuickClear is a simple and easy to use mechanical thrombectomy system,” said Bryan Fisher, M.D., chief of vascular surgery, Tristar Centennial Medical Center, Nashville, U.S. “The system is significantly smaller than other systems without compromising aspiration power. The convenience of the device really shines through with its single use and lack of capital equipment. I am excited about the potential of this device and the impact it will have on my practice and the patients I treat.”
QuickClear simplifies the entire thrombectomy procedure workflow. The small footprint of the sterile device allows it to be placed easily and conveniently on the table next to the patient. With the touch of a button, the system is up and running at maximum aspiration power within seconds. The consistency of the aspiration power during the procedure provides physicians more control and supports faster procedure times. The system’s range of catheters includes a large 10 French aspiration catheter, providing 59% more aspiration volume than 8F aspiration catheters.
Philips’ peripheral vascular portfolio already includes advanced interventional imaging systems for precision guidance; intravascular ultrasound (IVUS) catheters to assess the location of the disease and lesion morphology and guide and confirm the treatment; peripheral atherectomy devices to remove blockages; and peripheral therapy devices, such as Philips’ Stellarex drug-coated balloon, to treat lesions. Philips recently further extended this portfolio with the acquisition of Intact Vascular, maker of the Tack Endovascular System, a first-of-its-kind, minimal-metal, dissection repair device that provides precision treatment of peripheral arterial dissections following balloon angioplasty in above-the-knee (ATK) and below-the-knee (BTK) therapeutic interventions.
The Philips QuickClear Mechanical Thrombectomy System is U.S. FDA 510(k) cleared and available for sale in the U.S., with future expansion of availability to other geographies planned.


 November 14, 2025
November 14, 2025 









