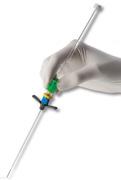
February 11, 2011 – A power-injectable, extended-dwell catheter has gotten the CE Mark. The Powerwand, from Access Scientific, is placed using the Accelerated Seldinger Technique, and promises to improve the inpatient experience while increasing worker safety.
"Vascular access specialists have a keen interest in the Powerwand, because they recognize patients' need for an extended dwell catheter that allows for medication/fluid delivery and also permits diagnostic phlebotomy – all through a single non-central line," said Steve Bierman, M.D., CEO of Access Scientific. “The wand’s proprietary technology is designed to make over-wire vascular access faster, safer and simpler. The device is designed to protect both patients and healthcare workers. With the Powerwand, many if not most inpatients can have one and only one needlestick during their entire hospitalization."
The Powerwand has not yet received FDA 510(k) clearance.
For more information: www.The-Wand.com

 November 13, 2025
November 13, 2025