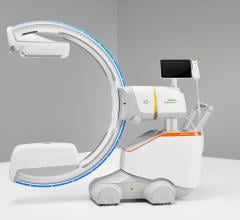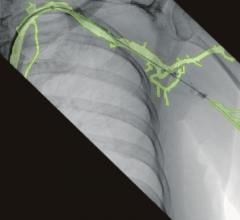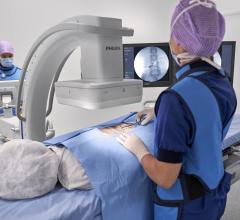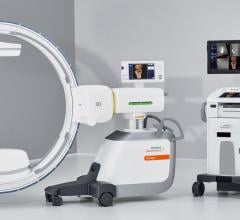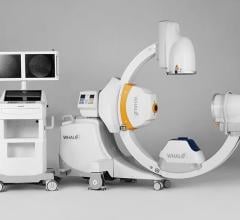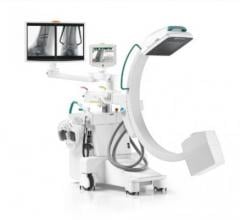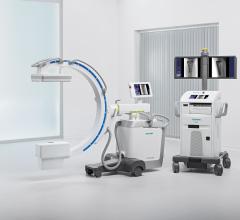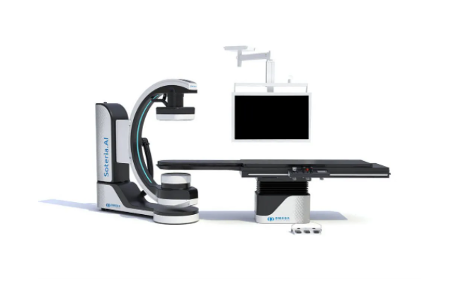
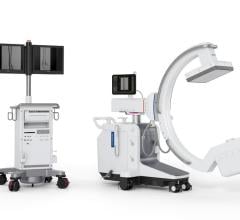
April 27, 2022 – Omega Medical Imaging has announced the release of the revolutionary Soteria.AI. Designed specifically for cardiology labs, Soteria.AI represents the next generational leap in image-guided X-ray systems. Combining the newest, most advanced technologies in medical imaging, Soteria.AI delivers the ultimate radiation protection – reducing dose by up to ~84% – with spectacular image quality in an exclusive, innovative design.
As the newest, most advanced system in medical imaging, Soteria.AI delivers the performance and safety that Omega is known for. An AI-enabled automatic ROI drives an ultra-fast secondary collimator that goes beyond mere filtering to provide the ultimate radiation protection. The patented design of Soteria.AI delivers the projections and patient access previously reserved for much larger, more costly ceiling-mounted systems. Soteria.AI delivers this safety and function with state-of-the-art image processing and unmatched image quality.
Soteria.AI provides several advantages that conventional cardiology systems cannot:
- Proven radiation protection for patients and staff
- Patented C-Arm design delivers ceiling-mounted performance
- Superior image processing and quality
“Soteria.AI introduces a new modality to cardiac imaging that creates a higher standard of care in interventional systems,” said Brian Fleming, President and CEO of Omega Medical Imaging. “AI-guided ROI systems bring safety and superior imaging to the lab and that’s a win for everyone – the hospital, the doctors, and the patient.”
The name Soteria.AI was chosen carefully. Soteria is the Greek goddess of safety and salvation, deliverance, and preservation from harm. This describes Soteria.AI perfectly with performance, reliability, and safety designed into every system.
To learn more, visit https://www.omegamedicalimaging.com/omega-soteria-ai/

 June 20, 2024
June 20, 2024