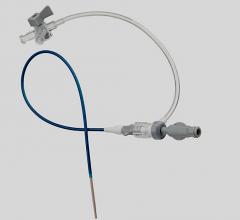
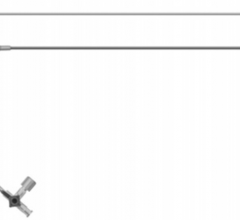
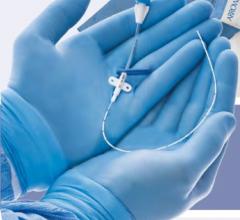
July 3, 2008 - A unanimous federal jury in Texas recently found that two patents exclusively licensed to Pressure Products Medical Supplies Inc., which cover medical products called valved peel-away introducers used for placement and implantation of pacemaker leads, were valid and infringed by the FlowGuard and ViaSeal products marketed by Quan Emerteq Corp. d/b/a Enpath Medical a division of Greatbatch Inc.
The court has set a hearing in July 2008 to determine whether to issue a permanent injunction barring any further marketing or sale of the FlowGuard and ViaSeal by Enpath.
Pressure Products said it is pleased with the decision and it will continue to sell its patented SafeSheath and DPRO products into the cardiac and dialysis markets.
For more information: www.pressure-products.com

 June 10, 2020
June 10, 2020