December 22, 2008 - W. L. Gore & Associates last week said Rush University Medical Center in Chicago is the first medical center to enroll a patient in the Gore REDUCE Clinical Study using the percutaneous GORE HELEX Septal Occluder to close patent foramen ovale (PFO).
“Rush University Medical Center is honored to be the first site to enroll a patient in the Gore REDUCE Clinical Study,” said the site’s study co-investigator Ziyad Hijazi, M.D., director of the Rush Center for Congenital and Structural Heart Disease and professor of pedatrics and internal medicine. “The reported experience with the device thus far from Europe has proven to be promising, so we are thrilled to be part of this multi-national trial which will help us further our understanding as to whether the GORE HELEX Septal Occluder significantly reduces recurrent embolic events as compared to medical treatment.”
Co-investigator Vivien Lee, M.D., stroke neurologist, said, “We are excited to be contributing to a trial that is geared toward determining whether there is a viable alternative to the current standard of care for the treatment of stroke patients.”
The U.S. principal Investigators for the Gore REDUCE Clinical Study are John Rhodes, M.D., chief of clinical cardiology at Duke University Medical Center, and Scott Kasner, M.D., associate professor of neurology and director of the stroke center at the University of Pennsylvania Medical Center. The study is a prospective, randomized, multi-center, multi-national trial designed to demonstrate safety and effectiveness of the GORE HELEX Septal Occluder for PFO closure in patients with a PFO and history of cryptogenic stroke or imaging confirmed transient ischemic attack (TIA). Patients are randomized to one of two treatment arms, either anti-platelet medical management alone or device closure of the PFO in conjunction with anti-platelet medical management. The primary endpoint is freedom from recurrent ischemic stroke, imaging confirmed TIA, or death due to stroke through 24 months post-randomization. The study sponsor is pursuing up to 50 participating investigational sites in the U.S. and Nordic countries.
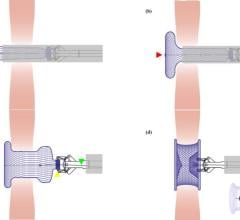
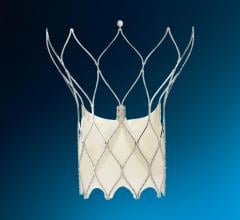
The GORE HELEX Septal Occluder was approved by the FDA last year for treatment of atrial septal defect (ASD), a congenital heart defect. The GORE HELEX Septal Occluder is the first device of its kind to use ePTFE, a biocompatible material that allows progressive tissue ingrowth, to help seal the defect. The GORE HELEX Septal Occluder has been designed to be soft and flexible, conforming to the anatomy of the heart while bridging and covering the defect to stop the shunting of blood between the atria.
For more information: www.goremedical.com


 June 20, 2024
June 20, 2024