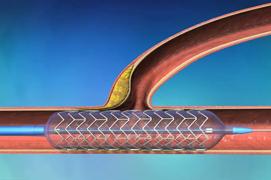
January 11, 2011 – TriReme Medical has signed an exclusive distribution and sales agreement with Century Medical for the Japanese market. Century Medical will sell TriReme’s compete line of advanced peripheral and coronary balloon dilatation catheters.
TriReme recently received clearance from The U.S. Food and Drug Administration (FDA) recently cleared the company’s GliderXtreme PTA balloon catheter. The highly differentiated balloon catheter features an expanded matrix of sizes including longer balloon lengths, an atraumatic tapered tip and shaft construction reinforced for torque transmission and exceptional pushability. It is designed to cross difficult and highly diseased lesions to restore blood flow in the complex peripheral anatomy. The coronary version of this product, known as the Glider PTCA balloon catheter is designed to overcome the limitations of conventional balloon catheters in treating complex disease by improving torque and push transmission.
For more information: www.trirememedical.com


 June 13, 2024
June 13, 2024 









