Doreen DeFaria Yeh, M.D., associate director, MGH Adult Congenital Heart Disease Program, and co-director, MGH ...
The Centers for Medicare & Medicaid Services (CMS) Center for Medicare and Medicaid Innovation (Innovation Center) announced the launch of a new voluntary bundled payment model called Bundled Payments for Care Improvement Advanced (BPCI Advanced). Under traditional fee-for-service payment, Medicare pays providers for each individual service they perform. Under this bundled payment model, participants can earn additional payment if all expenditures for a beneficiary’s episode of care are under a spending target that factors in quality.
In a new health policy statement, the American College of Cardiology (ACC) identifies how to best support healthcare advances in three arenas — digital health, big data and precision health.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 9, 2018 – Avinger Inc. announced that Arne Schwindt, M.D., a vascular surgeon at St. Franziskus Hospital in ...
Competitive male triathletes face a higher risk of a potentially harmful heart condition called myocardial fibrosis, according to research presented at the annual meeting of the Radiological Society of North America (RSNA), Nov. 26-Dec. 1 in Chicago. The increased risk, which was not evident in female triathletes, was directly associated with the athletes’ amount of exercise.
EchoPixel showcased the latest version of True 3D, its interactive, mixed reality software solution at the 103rd Annual Radiological Society of North America (RSNA) meeting, Nov. 26-Dec. 1, 2017, in Chicago. True 3D was featured in the Virtual Reality Showcase in the RSNA Learning Center.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
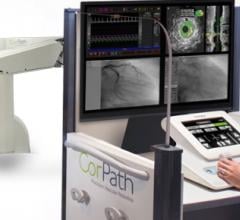
Corindus Vascular Robotics Inc. announced that it is working with Mayo Clinic in a preclinical study about use of telestenting. Telestenting, a remote robotic treatment for percutaneous coronary intervention (PCI), may enable physicians to conduct procedures from virtually any location, opening opportunities for more patients globally to receive the benefits of this lifesaving procedure. The global shortage of PCI-capable operators is significant and continues to be a growing problem.
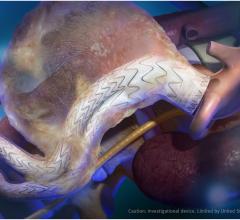
January 8, 2018 — W. L. Gore & Associates Inc. announced the first implant of the Gore Excluder Conformable AAA ...
Guerbet announced that it has entered into an agreement under which it will acquire Israeli company Accurate Medical Therapeutics , which specializes in the development of microcatheters used in interventional radiology.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...
January 8, 2018 — Canon Inc. and Canon Medical Systems Corp. announced the official corporate name change of Canon Group ...
Bernadette Speiser, BSN, MSN, CCRN, RCIS, a cardiac cath/EP nurse at Palo Alto Veterans Hospital, Palo Alto, Calif., and ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The U.S. Food and Drug Administration (FDA) announced a Class I recall of Sterilmed’s Agilis Steerable Introducer Sheath’s hemostatic valve that prevents blood from flowing back through the valve, warning that it may fail due to an improper seal of the sheath hub. The agency said improper seals can allow blood to leak through the hub, cause the cap to fall off during the procedure, or can create a difference in pressure that allows air into the circulatory system (air embolism). The FDA warned the use of affected products may cause serious health consequences for patients, including death.
Adam Greenbaum, M.D., co-director, Center for Structural Heart Disease, Henry Ford Hospital, Detroit, explains how his ...
Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labeling for the Quadra Assura MP cardiac resynchronization therapy defibrillator (CRT-D) and Fortify Assura implantable cardioverter defibrillator (ICD). The approvals follow recent MR-conditional labeling approvals for the Assurity MRI pacemaker, Ellipse ICD and associated MRI-compatible leads, and further expand Abbott's portfolio of MRI-ready devices for patients indicated for ICDs and/or CRT-D devices who may need an MRI in the future.

 January 11, 2018
January 11, 2018