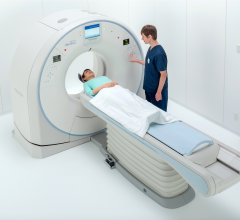
April 12, 2017 — Providers can now offer enhanced care and safe imaging to patients with a compact and economical ...
Biotronik announced the European launch of a new lead for tachycardia therapy that features a helical design for increased long-term performance through stress reduction. The Plexa ProMRI lead is made for use with implantable cardiac defibrillators (ICDs) as well as cardiac resynchronization therapy defibrillators (CRT-Ds).
Through a new clinical trial, patients at Harrisburg, Pa.-based PinnacleHealth have access to an investigational device designed to reduce risk of stroke during transcatheter aortic valve replacement (TAVR).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 11, 2017 — IBA Molecular announced that it has merged with previous acquisition Mallinckrodt Nuclear Medicine LLC ...
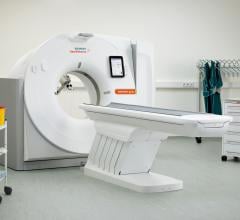
The U.S. Food and Drug Administration (FDA) has cleared the Somatom go. computed tomography (CT) platform from Siemens Healthineers, designed for highly diverse sets of user needs.
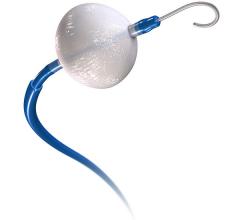
April 10, 2017 — Boston Scientific recently announced a $435 million cash deal to acquire Symetis SA, a privately-held ...
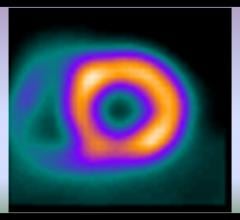
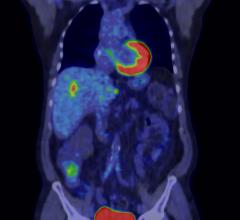
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 10, 2017 — Medtronic recently announced the first enrollments in the STOP Persistent AF clinical trial. The study ...
A discussion with Heart Rhythm Society (HRS) President Michael Gold, M.D., Ph.D., director of cardiology and associate ...
David Steenblock, D.O., an osteopathic physician based in San Clemente, Calif., recently discussed his use of hyperbaric oxygen to achieve significant and improved lifestyle outcomes for many stroke patients. The technique involves the therapeutic use of oxygen under pressure to dramatically reduce the effects of stroke and brain injury.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Endologix Inc. announced that the first patients were treated in the Expanding Patient Applicability with Polymer Sealing Ovation Alto Stent Graft (ELEVATE) IDE clinical study.
The Society of Cardiovascular Computed Tomography (SCCT) and Toshiba Medical announced a new partnership dedicated to providing career development opportunities for radiology fellows and residents in the United States. Toshiba Medical’s support will allow 250 U.S.-based fellows and residents to join SCCT at no charge for one year, enhancing their early career opportunities and building their awareness of the growing contributions of cardiovascular computed tomography.
PTS Diagnostics announced that the U.S. Food and Drug Administration (FDA) has cleared an expanded top range for measurement of HDL cholesterol (HDL) to 120 mg/dL (3.1 mmol/L) for the company's PTS Panels lipid panel test strips. The PTS Panels lipid panel test strip now has a measurement range of 20 to 120 mg/dL (0.52 to 3.1 mmol/L) for HDL. Additionally, the lipid panel shelf life has increased due to the use of new test strip vials.
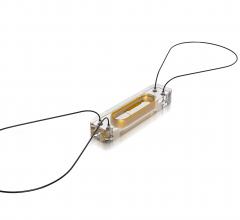
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
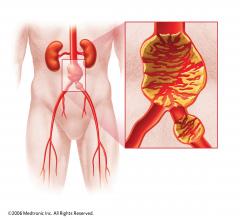
Following repair of abdominal aortic aneurysms (AAA), women appear to have more unfavorable outcomes than men in terms of mortality and morbidity.
April 5, 2017 — GE Healthcare has signed an agreement with HealthTrust, a group purchasing organization headquartered in ...
This week the Centers for Medicare & Medicaid Services (CMS) convened a panel of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) to examine which health outcomes in studies of heart failure treatment technologies should be of interest to CMS. The panel also assessed the growing challenges associated with the changing landscape of evidence generated prior to market authorizations of new and innovative technology. While MEDCAC panels do not make coverage determinations, CMS does benefit from their guidance.

 April 12, 2017
April 12, 2017