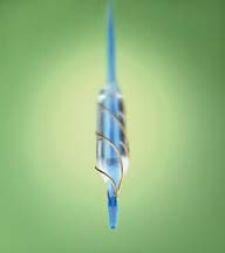
Joining a new class of specialty medical devices aimed at treating peripheral arterial disease (PAD), the AngioSculpt Scoring Balloon Catheter from AngioScore Inc. has the potential to favorably improve the clinical outcomes of a large portion of these patients.
The catheter is a line of innovative coronary and peripheral angioplasty catheters comprising an angioplasty balloon surrounded by a unique system of nitinol scoring elements. Creating focal concentrations of dilating force, the scoring elements score arterial lesions as the balloon expands.
The line was developed to address the clinical need for a safe, high-performance predilatation catheter that also provides the benefits of plaque scoring in complex coronary cases such as those involving calcified lesions, ostial lesions, small vessels and in-stent restenosis. Combining catheter deliverability with the benefits of plaque scoring and a high-rated burst pressure (up to 20 atm), the scoring balloon catheter will be a valuable tool for complex lesions.
Distribution of the AngioSculpt in the U.S. is expected to begin in the first quarter of 2006.


 October 28, 2025
October 28, 2025 









