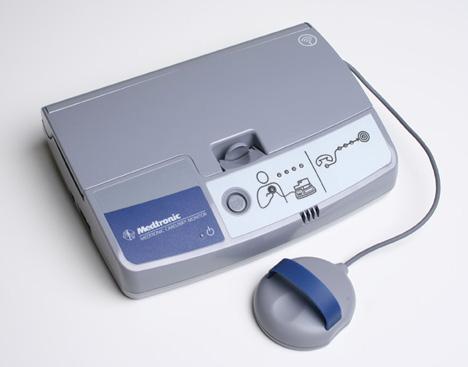
May 11, 2010 – A cellular accessory has been added to an implantable cardiac device remote monitoring system to make it easier for patients to securely send information to their physicians. The Medtronic M-Link allows transmission of information via the CareLink Network using cellular signals, rather than a telephone landline.
The cellular accessory makes remote monitoring accessible to a greater number of eligible patients. It also provides patients with a convenient option to stay connected with their clinic from home, work, or while traveling globally. The device allows Medtronic CareAlert notifications to be transmitted when any of the programmable alert conditions from a patient’s implanted device has occurred. Physicians and nurses can view the transmitted data through a secure Web site, giving them the opportunity for a real-time look at how the patient’s device is functioning.
Medtronic will showcase the M-Link cellular accessory May 12-15 at Heart Rhythm 2010 in Denver, booth 503.
The introduction of the M-Link cellular accessory comes on the heels of a landmark study showcasing the benefits of remote monitoring for patients and their physicians. Results from Medtronic’s CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial presented at the American College of Cardiology Scientific Sessions in March 2010 showed cardiac resynchronization therapy-defibrillator (CRT-D) and implantable cardioverter-defibrillator (ICD) patients monitored through the CareLink Network experienced a significant reduction in time from clinical event to clinical decision and also benefited from shorter hospital stays and reduced costs per hospitalization.
For more information: www.medtronic.com


 July 21, 2025
July 21, 2025 









