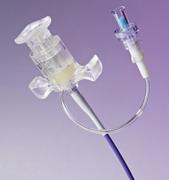
May 13, 2010 – An integrated, break-away hemostasis valve is integrated into a new lead delivery system is designed to minimize blood loss and allow for easier implantation. The U.S. Food and Drug Administration (FDA) cleared the Acuity Break-Away Lead Delivery System for use with cardiac resynchronization therapy defibrillators (CRT-Ds) and cardiac resynchronization therapy pacemakers (CRT-Ps).
The delivery system is designed to place leads in veins of varying sizes, including those difficult-to-access. It is supposed to simplify the implant experience for both patients and physicians.
Key features of the Acuity Break-Away Lead Delivery System include:
• An integrated, break-away hemostasis valve designed to minimize blood loss and allow for a streamlined implant experience with fewer steps and instruments used during surgery
• A very small inner catheter to deliver a 4 French lead
• Seven outer and two inner guide catheter shapes designed to deliver the lead in a variety of anatomies
Boston Scientific’s left ventricular leads include four designs that provide options for securing the lead to the heart. This lead portfolio also features Electronic Repositioning, which provide six configurations for stimulating the left side of the heart, even after implant, potentially avoiding an additional surgical procedure.
For more information: www.bostonscientific.com


 July 21, 2025
July 21, 2025 









